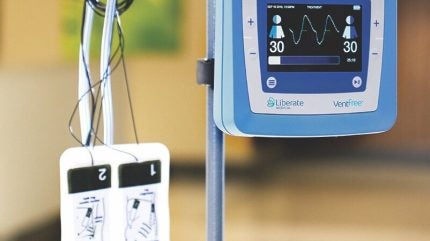
Liberate Medical has commenced patient enrolment in a study investigating its neuromuscular electrical stimulator to help respiratory failure patients wean off mechanical ventilation.
The study, named PREVENT, is being funded by the US Department of Defense (DOD). The organisation awarded $6.8m to Liberate through the Peer Reviewed Medical Research Programme.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PREVENT is a randomised, sham-controlled, blinded trial that plans to enrol 272 patients across the US, European Union, and Australia.
Liberate’s device, called the VentFree Respiratory Muscle Stimulator, non-invasively reduces mechanical ventilation duration by improving expiratory muscle strength and cough.
The number of patients requiring long-term invasive mechanical ventilation is rising. There is a major burden for patients using assistive technology, with high costs for healthcare systems also present.
According to Liberate, VentFree is designed to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation and post-removal of breathing assist tubes.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe primary objective of the trial is to determine whether the device, via abdominal functional electrical stimulation, can reduce how long critically ill respirate failure patients spend on mechanical ventilation compared to sham control.
The therapy will be applied for half an hour, twice a day for at least five days a week. This will occur over 28 days or until the patient is discharged from intensive care.
The study’s co-global principal investigator Dr Leo Heunks said: “The expiratory muscles are a critical yet underappreciated component of respiratory health, and this trial will highlight their significance.”
The PREVENT trial follows two pilot studies completed in Europe and Australia, which demonstrated improved expiratory function and reduced ventilator duration and intensive care unit stay length.
VentFree has breakthrough device designation and emergency use authorization from the US Food and Drug Administration (FDA), as well as CE marking in the US and EU respectively.
Liberate raised $6.2m in a Series B financing round in July 2023, adding to a previous total of $1.45m raised by the company through venture financing, according to GlobalData’s deals database.
The global neuromodulation device market is expected to reach $11.4bn by 2033, according to a report by GlobalData.





