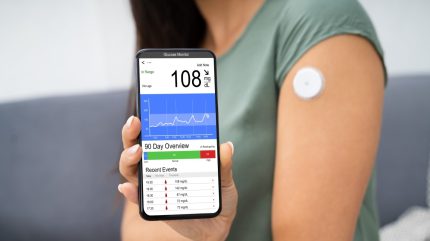
Senseonics, in collaboration with Ascensia Diabetes Care, has announced that its Eversense product received an integrated continuous glucose monitoring (iCGM) designation from the US Food and Drug Administration (FDA).
This marks Eversense as the first fully implantable device to achieve such status, paving the way for future devices of its kind through the FDA’s De Novo pathway.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The iCGM designation signifies that the system can be integrated with compatible medical devices, such as insulin pumps, to create an automated insulin delivery (AID) system.
With this integration, Eversense can now address the common limitations of AID systems identified in the 2022 Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association.
Senseonics president and CEO Tim Goodnow said: “The iCGM designation has been a core component of our strategic initiatives to advance our pipeline, and we are excited to move forward with the next steps to accelerate the integration of the world’s longest-lasting CGM with leading insulin devices.
“The rigorous data requirements for this FDA designation highlight our team’s advanced engineering expertise in developing a CGM that meets high standards. Our confidence in our differentiated technology is high, as there is a tremendous opportunity with iCGM to deliver value and provide substantial benefits to diabetes patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“As we look ahead, we are focused on progressing our partnership discussions and software developments, and look forward to providing more updates.”
Senseonics and Ascensia are now set to advance partnership discussions with insulin pump manufacturers, aiming to provide a new interoperable CGM option for those integrating their diabetes management devices.
The Eversense CGM System, designed by Senseonics and distributed by Ascensia, is distinguished by its six-month wear time, accuracy in low glucose ranges, reliable alert detection, and a removable transmitter that does not require sensor wastage or additional warm-up time upon reattachment.
Last September, Senseonics completed the adult cohort of the ENHANCE pivotal clinical trial for Eversense, with the final study subject completing a 365-day visit.
The ENHANCE trial aimed to evaluate the safety and accuracy of the Eversense system over one year.





