Blue Earth Diagnostics has received approval from the US Food and Drug Administration (FDA) for its Posluma (flotufolastat F 18) injection.
Posluma is claimed to be the first and only radiohybrid (rh) prostate-specific membrane antigen (PSMA)-targeted positron emission tomography (PET) imaging agent to be approved by the FDA.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
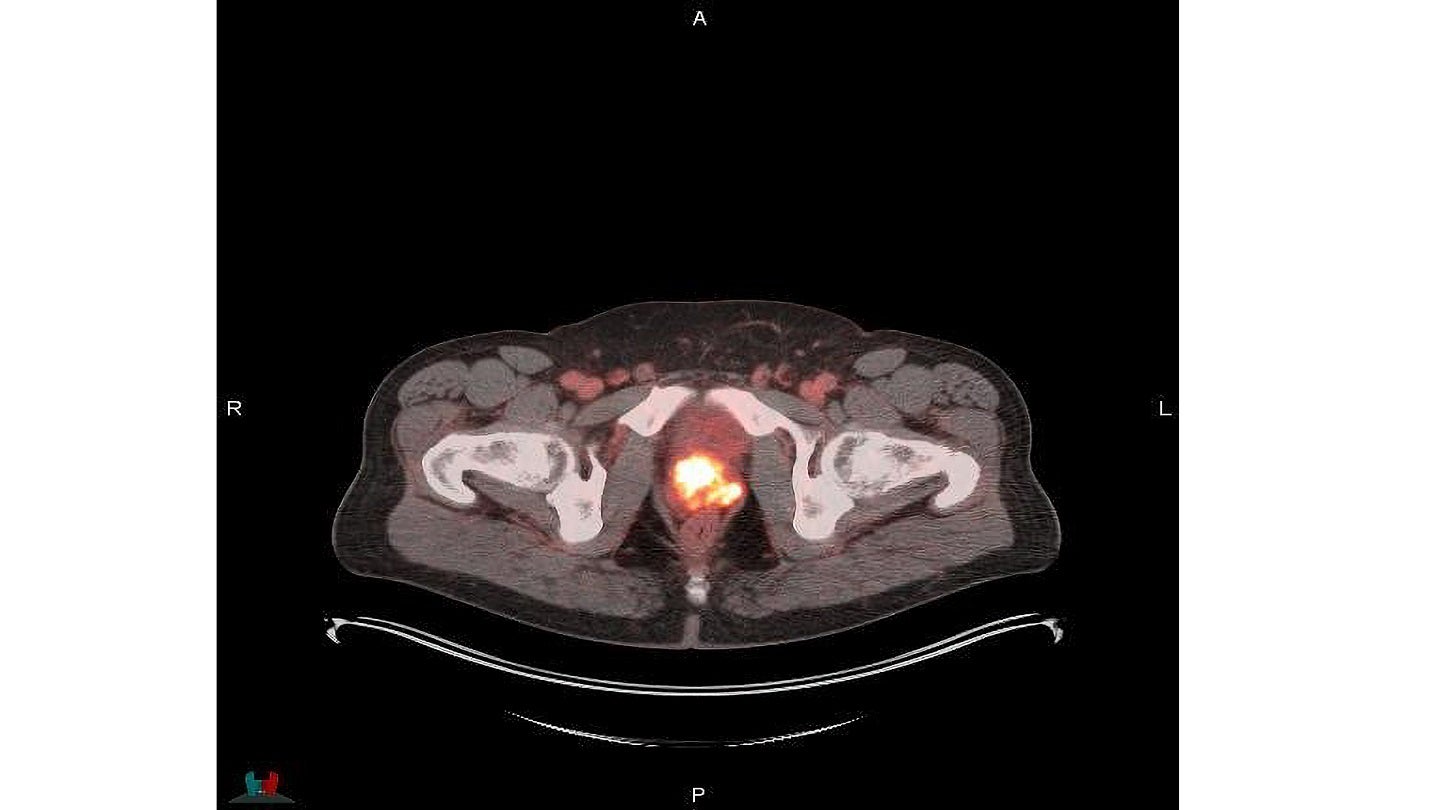
It is a radioactive diagnostic agent designed to be administered intravenously for PET of PSMA-positive lesions in adult male patients with prostate cancer.
The agent was developed to help physicians to identify and localise prostate cancer.
Posluma is intended for patients with suspected prostate cancer metastasis who are eligible for initial definitive therapy or with suspected recurrence based on increased serum level of prostate-specific antigen (PSA).
It is an optimised, PSMA-targeted rh diagnostic imaging agent that binds to and is internalised by PSMA-expressing cells.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe radioisotope fluorine-18 (18F) utilises the high image quality of 18F-labeled PSMA PET imaging to enable the effective identification of disease.
Following the approval, the company plans to commercially launch the product next month.
Blue Earth Diagnostics CEO David Gauden said: “With the FDA approval of Posluma, we realise our goal of providing an important product that will be widely available across the United States to help inform the management and treatment of patients across the prostate cancer care continuum.
“This event marks a major milestone in the expansion of Blue Earth Diagnostics’ robust prostate cancer portfolio.”
The FDA approval was granted based on results from two Phase III trials named LIGHTHOUSE and SPOTLIGHT.
The studies were designed to evaluate the safety and performance of Posluma across the spectrum of prostate cancer care.
The LIGHTHOUSE study showcased that the diagnostic imaging agent has high specificity for the detection of pelvic lymph nodes compared to histopathology truth standard in men with PSMA-positive lesions prior to undergoing treatment with radical prostatectomy.
Results from the SPOTLIGHT study indicated that Posluma has high detection rates even at low PSA levels.



