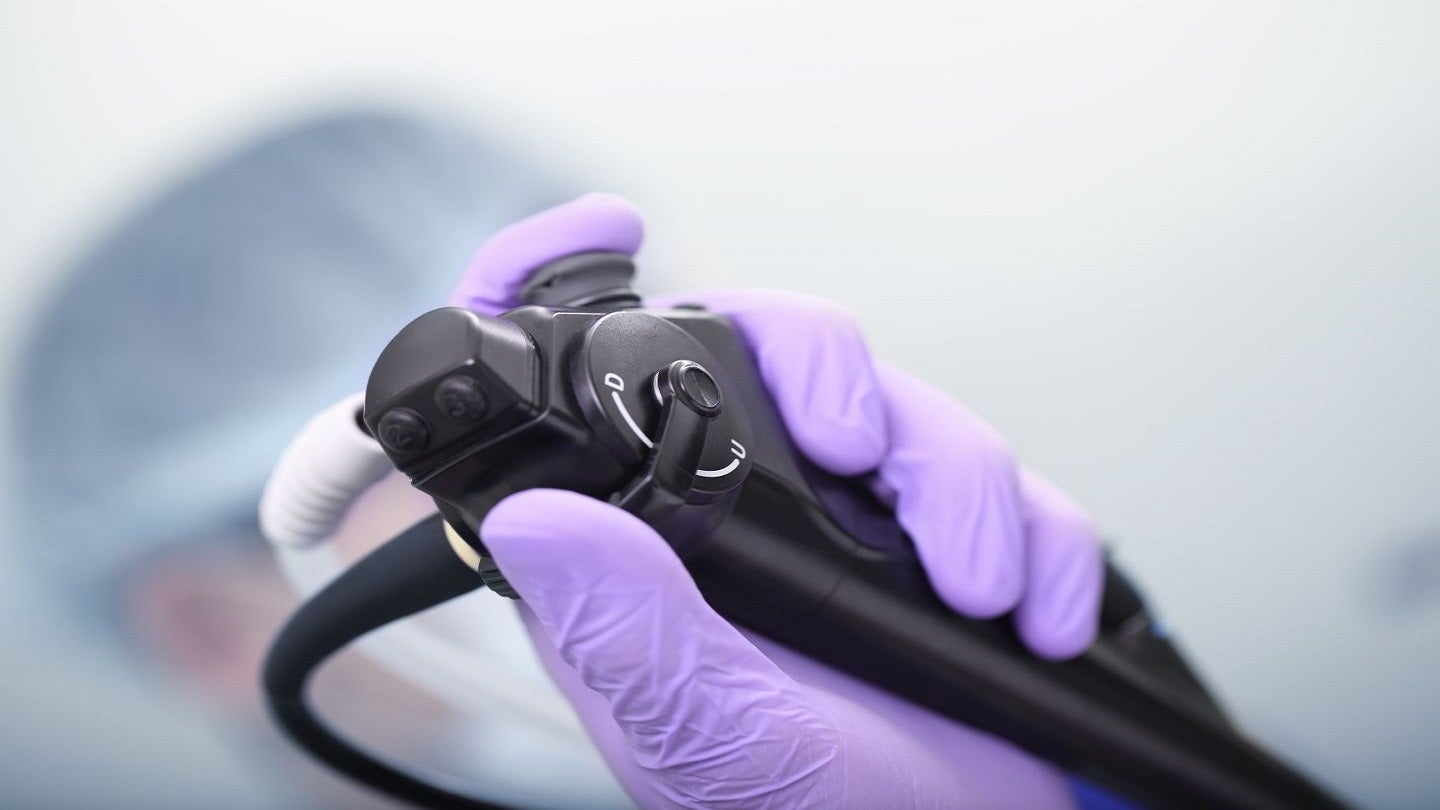
Olympus Corporation of the Americas (Olympus) has issued a voluntary labelling update for bronchoscopes used with laser therapy equipment.
The voluntary field corrective action is intended to address complaints of endobronchial combustion occurring while using laser-compatible bronchoscopes at the time of therapeutic procedures along with argon plasma coagulation (APC) or laser therapy equipment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The corporation has included 32 BF series endoscope models in this global action, with 19 of these models being supplied in the US.
Olympus has notified this action to the US Food and Drug Administration and other regulatory bodies.
The company’s bronchoscopes are suitable for use in endoscopic diagnosis and treatment within the airways and the tracheobronchial tree.
It evaluated the issue after receiving adverse event complaints, including one death and patient injury.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe corporation also determined that updated labelling was required to specify laser compatibility and warn about the risks associated with incompatible laser use as well as bolster existing warnings.
Olympus received a complaint about endobronchial combustion with APC but the cause related to the event could not be determined. It is also not implementing any updates to the labelling regarding the use of APC.
The company did not assess any other lasers for compatibility with the indicated bronchoscope models.
In addition, the corporation suggests that users must carefully study warnings in the operation manual on laser cauterisation with its bronchoscopes.



