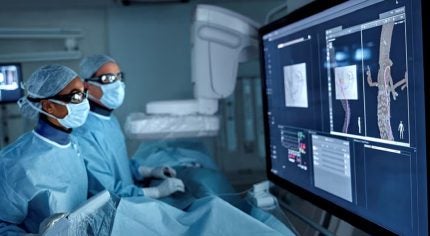
Philips has introduced LumiGuide, which utilises Fiber Optic RealShape (FORS) technology to enable minimally invasive surgery without the need for X-ray radiation.
The innovation is known as the “3D human GPS powered by light”.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The technology has been developed in collaboration with clinical partners and is initially available to major Aortic Centers of Excellence in the US and Europe.
It works by reflecting light along an optical fibre inside a guidewire, creating 3D, high-resolution, colour images of devices inside the body in real-time.
This allows physicians to see the direction of their device and navigate to the required location without X-ray assistance.
Philips has reported promising results from studies conducted around the world.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMore than 900 patients have undergone procedures using LumiGuide, with one site demonstrating a 37% reduction in complex aortic procedure time and a 56% reduction in radiation exposure compared to X-ray.
LumiGuide is designed to be compatible with Philips interventional systems such as Azurion.
Maastricht University Medical Center in the Netherlands vascular surgeon Geert Willem Schurink performed the first surgical procedure with LumiGuide.
Schurink said: “This AI-based semi-automatic registration is very quick and accurate, even in the presence of stent grafts. Especially, if there is a need to re-register the device being guided in the patient’s body during the procedure, it is extremely helpful.”
As Philips and its clinical partners continue to collect data on LumiGuide’s performance, the company is preparing to make the solution available globally.
Recently, Philips obtained 510(k) clearance from the US Food and Drug Administration for its latest transoesophageal echocardiography transducer to help improve cardiac care for more patients.





