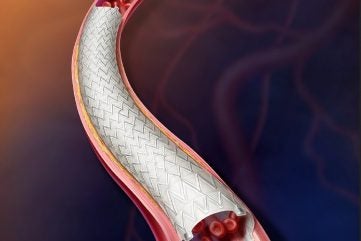
Becton, Dickinson and Company (BD) has enrolled the first subject in the investigational device exemption study, AGILITY, using its Vascular Covered Stent to expand treatment options for peripheral arterial disease (PAD).
The study will evaluate the safety and effectiveness of the BD Vascular Covered Stent, a low-profile, self-expanding, polytetrafluoroethylene encapsulated nitinol implant.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The AGILITY clinical study is a global, prospective, multi-centre, single-arm, non-randomised investigation.
It will involve 315 patients at up to 40 trial sites across the US, Europe, New Zealand and Australia. Patients will be followed up at various points post-treatment, from one month up to 36 months.
For the trial, the company enrolled the first patient at Trinity Medical Center in Bettendorf, Iowa, US.
Cardiovascular Medicine interventional cardiologist; Midwest Cardiovascular Research Foundation founder and president; and University of Iowa Adjunct professor of Medicine Dr Nicolas Shammas led the enrolment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Vascular Covered Stent is deployed from a delivery system designed for controlled stent release.
Cleveland Clinic Department of Vascular Surgery chairman and the AGILITY study national principal investigator Dr Sean Lyden said: “When we’re addressing advanced PAD, a self-expanding covered stent can play an important role.
“We need a stent that can track the lesion, apposes the vessel wall and ultimately provides long-term durability. We’re excited to see how this technology performs.”
BD Peripheral Intervention vice-president and general manager Tim Hug said: “There continues to be significant unmet needs in the treatment of PAD patients.
“We are excited to have initiated this study and evaluate the treatment benefits of this potentially differentiated technology.
“This stent could give interventionalists an important new solution in the fight against PAD, expand our portfolio and enable us to better serve our customers and the patients they treat.”
Recently, BD partnered with Camtech Health in Singapore to introduce a self-collection human papillomavirus (HPV) test for women to use at home.





