myBiometry has secured a $5m investment in an oversubscribed seed funding round to propel the development of its fenoTRACK device for asthma and chronic obstructive pulmonary disease (COPD).
The funding round was spearheaded by Dexcom Ventures and saw contributions from Elevate Ventures and CareSource Indiana, among other investor groups.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
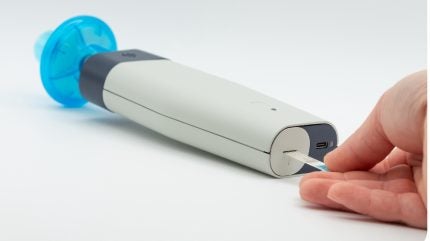
This financial boost is set to advance the fenoTRACK technology, a portable device that aims to empower patients with critical data for the early detection of exacerbations.
fenoTRACK operates as a non-invasive breath test that quantifies type 2 airway inflammation by measuring fractional exhaled nitric oxide (FeNO).
Type 2 inflammation is identified as a primary cause in 80% of asthma cases and 30%-40% of COPD cases.
The data obtained from FeNO measurements are crucial in identifying patients at heightened risk of an exacerbation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, personalising treatment based on FeNO data can diminish the risk of exacerbations by 38%-50% in adults and children, respectively.
myBiometry CEO Bryan Nolan said: “We’re excited to partner with Dexcom Ventures and CareSource as we near the milestone of submitting fenoTRACK for regulatory clearance.
“With enhanced at-home monitoring for asthma and COPD, we strive to identify attack risks sooner, enabling clinicians to offer more personalised care and preventive interventions that lessen the burden on patients, caregivers, and healthcare payers.”
CareSource Midwest regional vice-president Steve Smitherman said: “The impact of asthma and other lung diseases on the health and wellbeing of our members of all ages cannot be overstated.
“CareSource is constantly looking for innovations that can help our members better manage chronic conditions, which is why we are enthusiastic about Biometry’s work and a proud investor.”
The company is currently channelling its efforts towards finalising the development of the device and is also preparing for its regulatory submission.





