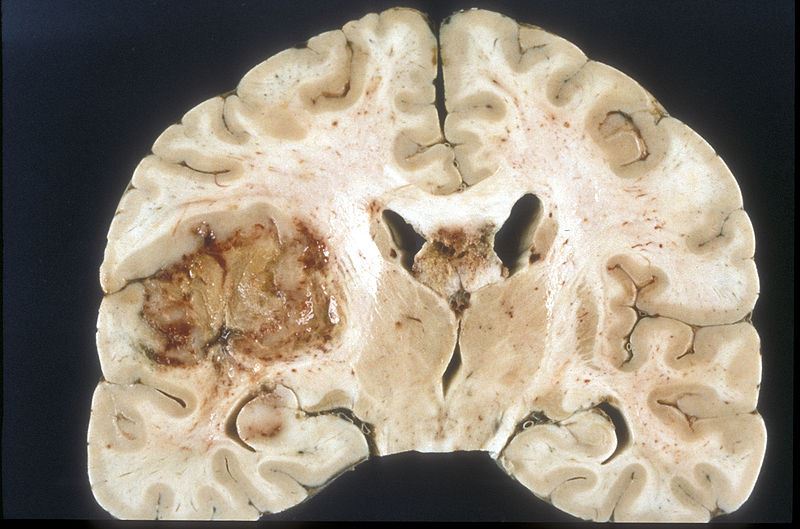
US-based Lantheus Holdings has entered into a strategic partnership with France-based CarThera to expand the use of its microbubbles for treatment of glioblastoma, an aggressive form of brain cancer.
The agreement is part of Lantheus’ strategy to expand its microbubble franchise into the field of oncology.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Under the agreement, Lantheus’ microbubbles will be used in combination with CarThera’s patented SonoCloud System.
SonoCloud is an implantable device in development to treat recurrent glioblastoma. It leverages low-intensity pulsed ultrasound (LIPU) to facilitate optimal penetration of chemotherapy drugs in the brain.
As part of the agreement, CarThera will handle all regulatory filings, approvals and commercialisation of SonoCloud in the US and Europe, as well as other parts of the world.
Lantheus will supply its microbubble vials, ultrasound resonator and activation devices at a predetermined price.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBased on regulatory approvals, Lantheus will also receive royalties on future sales of the SonoCloud kit.
Lantheus president and CEO Mary Anne Heino said: “We are excited to collaborate with CarThera to extend our microbubble franchise into the oncology field to target glioblastoma, an aggressive type of brain cancer with significant unmet medical need,”
“Our collaboration leverages both companies’ strengths to bring novel solutions to the healthcare community. As the use of microbubbles in diagnostic and therapeutic applications gains more interest around the world, our collaboration with CarThera demonstrates our commitment to drive Lantheus’ microbubble into new disease areas with great potential for significant improvement in patient outcomes.”
Currently, CarThera’s implantable ultrasound device SonoCloud-9 System is under the Phase IIa clinical trial evaluation process.
The trial, conducted among 21 patients, will evaluate the safety and efficacy of SonoCloud-9 to cover the entire tumour and surrounding infiltrative areas.





