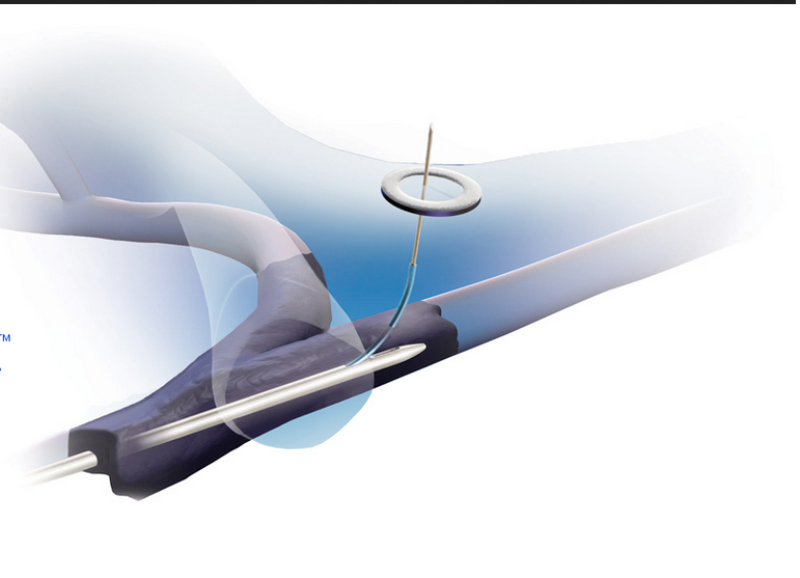
The US Food and Drug Administration (FDA) has granted a De Novo clearance to Surfacer Inside-Out Access Catheter System developed by private medical technology company Bluegrass Vascular Technologies.
The surfacer system, which employs a novel inside-out approach, is the first FDA-cleared medical device that facilitates upper body central venous access for patients with venous obstruction.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The approval comes from results of SAVE-US (Surfacer System to Facilitate Access in VEnous Occlusions – United States) trial.
SAVE-US lead principal investigator St Joseph Hospital interventional radiologist Mahmood Razavi said: “The surfacer system offers a safe and effective approach to reliably preserve and restore critical upper body vascular access sites.
“This is an unmet clinical need for patients who require life-saving therapies, such as dialysis, and who have limited options due to venous obstructions.”
The trial confirmed that, of the 30 enrolled patients, 90% met both primary and secondary efficacy endpoints, with no adverse events related to the device reported.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe current approach to treat venous obstruction is to use an alternative vein. This process results in increased catheter days and costs due to the reduced ability to place and mature a functioning fistula.
Bluegrass Vascular CEO and president Gabriele Niederauer said: “We are thrilled BVT has reached this important milestone. For the first time ever, physicians in the US can offer patients a reliable and repeatable solution to treat central venous obstructions and restore access to the right internal jugular vein, the preferred access site.
“Through our experience in Europe and other international sites, the Surfacer System has consistently demonstrated a positive clinical impact. We are eager to bring the Surfacer System and its important benefits to patients in the US.”
Bluegrass Vascular Technologies will introduce the surfacer system to select US centres in the coming months.
Earlier in 2016, the device received a CE mark. It is available in Europe, Canada, Singapore and the Middle East.





