Visit our Covid-19 microsite for the latest coronavirus news, analysis and updates
Follow the latest updates of the outbreak on our timeline.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
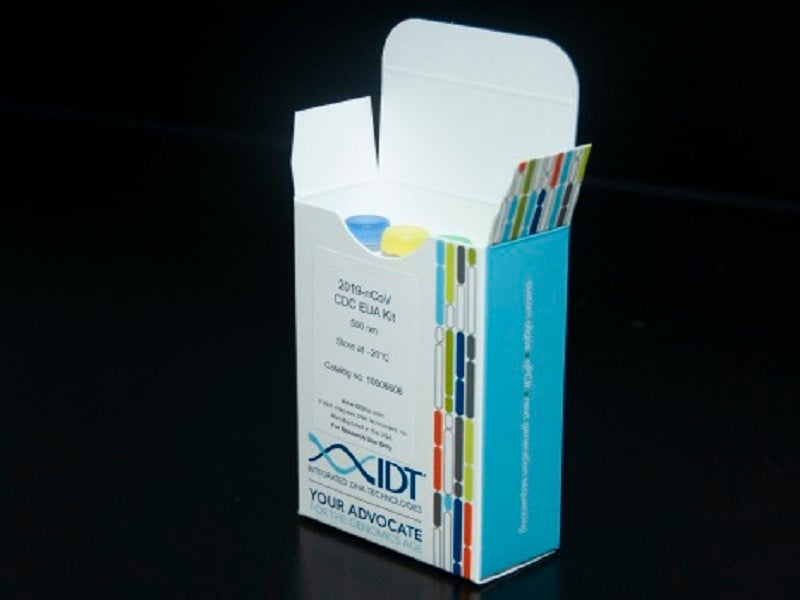
Integrated DNA Technologies (IDT) has announced its success in large-scale manufacturing of a fundamental Covid-19 component, which assists in DNA analysis of patient samples.
The company’s component is a primer and probe kit qualified by the Centers for Disease Control and Prevention (CDC).
Pursuant to the CDC Emergency Use Authorization (EUA) testing protocol, the company has shipped primer and probe kits, sufficient to enable more than one million coronavirus tests to be conducted.
Last week, IDT manufactured sufficient primer and probe kits to enable an additional 2.5 million tests.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt expects to manufacture five million tests per week starting from this week.
IDT President Trey Martin said: “We are honoured to be the first company in the nation to have our primer and probe kits approved by the CDC for use as a key component of the CDC EUA testing protocol for the diagnosis and detection of Covid-19.”
Previously, IDT provided products to diagnostic test manufacturers developing the test for H1N1, Ebola virus, and Zika virus.





