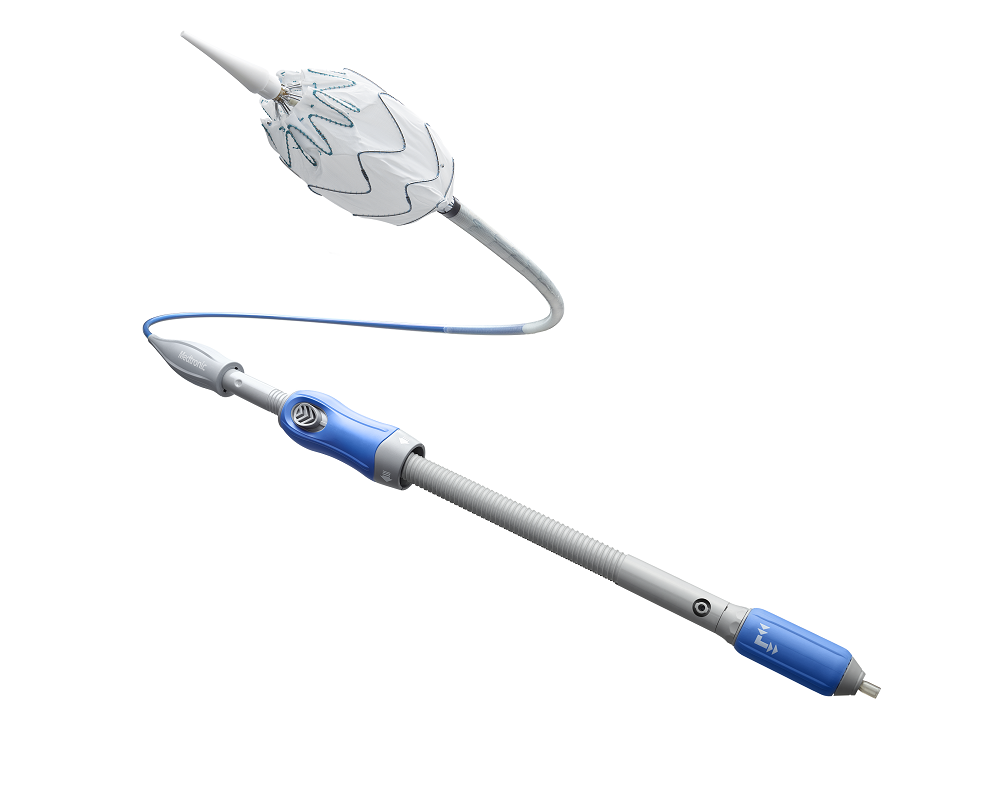
US-based medical technology company Medtronic has begun the post-market study of the Valiant Navion thoracic stent graft system in patients with thoracic aortic dissection.
The prospective, observational, global, multi-centre, real-world study will evaluate the safety and effectiveness of the system in the treatment of thoracic aortic dissection.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Thoracic aortic dissection is a serious condition, in which the inner layer of the lower aorta is torn, separating the inner and middle layers of the aorta.
The first patient procedure in the DISSECT-N study was performed at Northwell Health in New York by aortic surgery director Derek Brinster.
Approximately 200 patients with an acute or chronic thoracic aortic dissection will be enrolled in the study, which will take place across nearly 45 sites in North America, Europe and the Asia Pacific.
The primary endpoint is composite safety and effectiveness, including technical procedure success and freedom from major adverse events (MAEs) reported up to one month following the index procedure. Patients will be followed for a period of three years.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUniversity of Chicago Medicine Center for Aortic Diseases director Ross Milner and Netherlands-based St Antonius Hospital cardiothoracic surgeon Robin Heijmen are the primary investigators of the DISSECT-N study.
Medtronic Cardiac and Vascular Group aortic business vice-president and general manager John Farquhar said: “There remains a significant clinical unmet need in treating thoracic dissection, and we are dedicated to improving the care for these patients through an endovascular approach.
“With the DISSECT-N study, we hope to further support the Valiant Navion system as a less invasive approach to treating this condition.”
DISSECT-N study US principal investigator Ross Milner said: “I believe the DISSECT-N study – one of the larger prospective aortic dissection repair studies, which includes independent core-lab imaging review – will offer critical contemporary insights about TEVAR use in patients with various types of thoracic aortic dissection.”
The low-profile Valiant Navion system received the US Food and Drug Administration (FDA) approval and CE Mark in 2018.
It was also approved by the Ministry of Health, Labour and Welfare (MHLW) in Japan in September last year.





