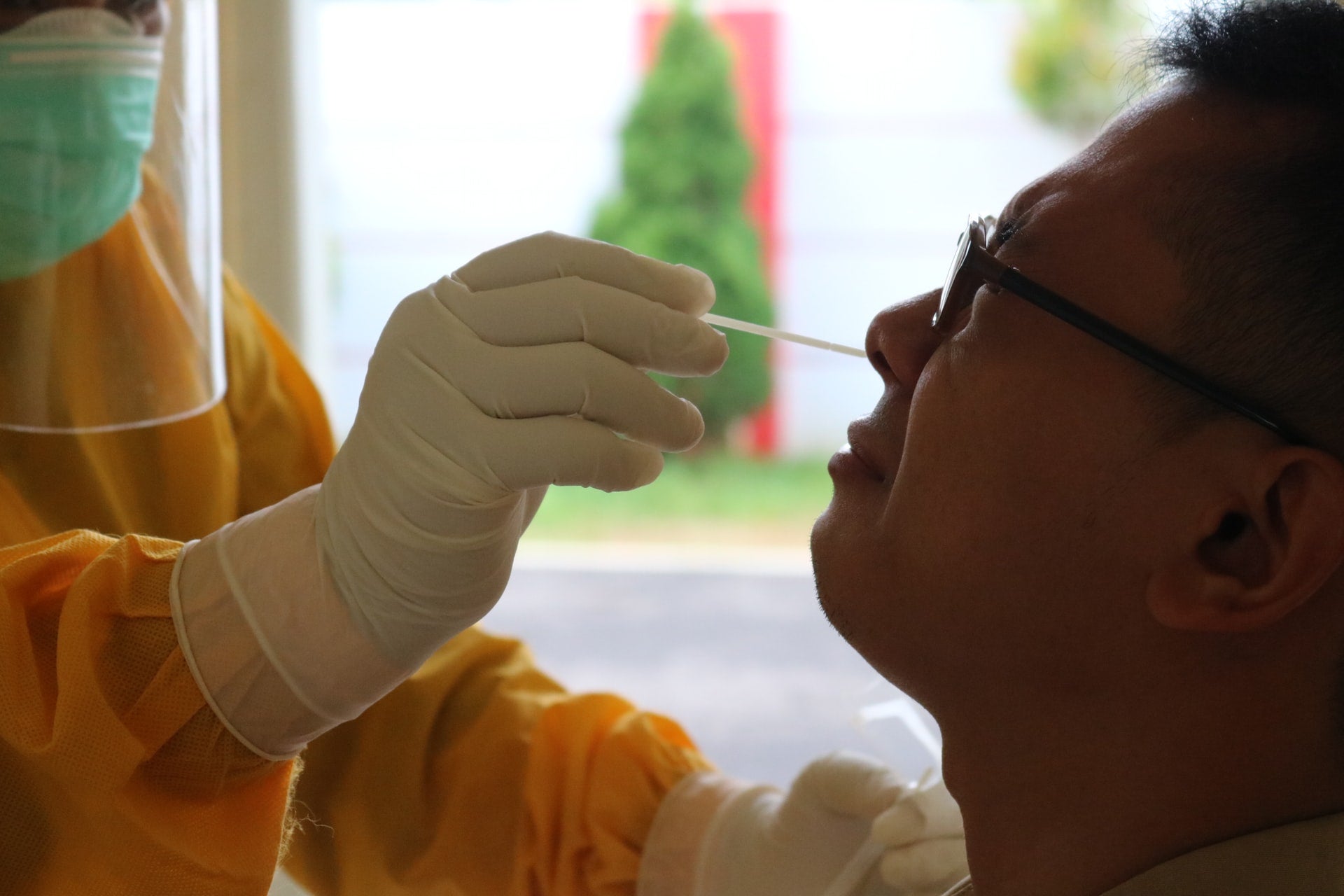
Sorrento Therapeutics has announced that the initial testing of its COVISTIX Covid-19 virus rapid antigen detection test showed its ability to detect Omicron besides the original SARS-CoV-2 virus and other major variants of concern.
The COVISTIX detection levels were found to be similar for all variants of concern.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company stated that recently a patient in Mexico infected with the Omicron variant was identified first using COVISTIX test in about 15-minutes.
This result was confirmed by RT-PCR test a day later and then sequence verified a few days later.
The company stated that in a laboratory setting, COVISTIX was able to detect the Omicron N protein at a significantly lower level compared with other Covid-19 rapid antigen tests that were Emergency Use Authorization (EUA) approved and commercially available.
The test also showed 20% higher sensitivity compared with a leading global brand in an in-field, real-world clinical study involving all participants including asymptomatic patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFrom a shallow nasal swab sample, the COVISTIX Covid-19 virus rapid antigen detection test can detect single SARS-CoV-2 virus antigens in 15-20 minutes, according to the company website.
Sorrento stated that it has capability to produce 30 million COVISTIX tests per month.
The company is also constructing a new production facility in San Diego, California, US. The facility will feature an automated assembly line with a capacity to produce six million COVISTIX tests per month.
The new facility will commence operations in the first quarter of next year.
To accommodate the accelerating global demand, Sorrento is planning to expand its COVISTIX tests production to 50 to 100 million per month next year.
Currently, the antigen test is CE marked in Europe, and is approved and marketed in Brazil and Mexico.
In a separate development, Sorrento’s Mexican subsidiary has executed a second contract to sell and distribute up to 10 million COVISTIX tests for the Mexican market.
The latest deal follows a contract with a local distributor for up to five million tests in Mexico.
Recently, the Indian Council of Medical Research (ICMR) developed a new test kit that has capability to detect the Omicron variant.





