The US Food and Drug Administration (FDA) has granted 510(k) clearance for the marketing of Sky Medical Technology’s new (W3) geko device variant.
The new device has been designed to increase microcirculatory blood flow in lower limb soft tissue in venous insufficiency and/or ischemia patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Last year, the company also received FDA approval for its (W2) geko device for venous insufficiency and/or ischemia, which is related to the reduced flow of blood in the veins and arteries.
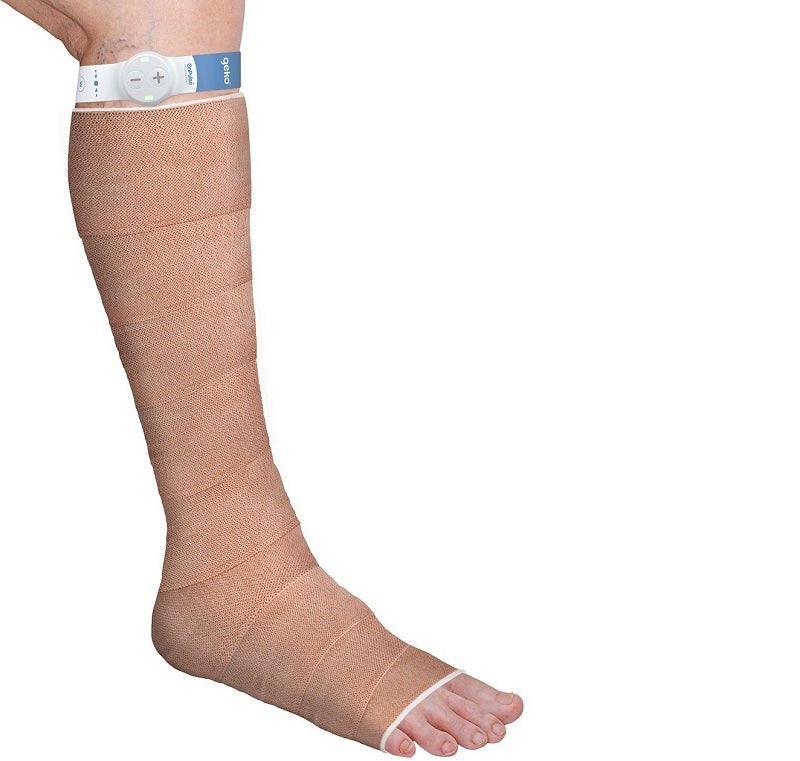
Non-invasive, wearable and easy to use, the geko device is the size of a wristwatch and can be worn at the knee.
It gently stimulates the common peroneal nerve and activates the calf and foot muscle pumps, which increases blood flow in the deep veins of the calf.
The company stated that the disposable device operates without the need for any external leg pressure and allows complete mobility.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt noted that the new (W3) geko device variant includes a third electrode, which enables significant nerve stimulation, as well as improved patient outcomes and comfort.
Additionally, the new device variant increases to two 12-hour therapeutic doses, from the two six-hour doses that the (W2) device provided.
Sky Medical Technology CEO and founder Bernard Ross said: “Achieving this latest 510(k) clearance for the geko device (W3) establishes Sky’s position as a leading innovator in MedTech dedicated to improving patient outcomes.
“Conditions such as venous insufficiency and ischemia are therapy areas sorely in need of innovation, and we are pleased to contribute to improvements in this care pathway that make recovery quicker, simpler and more comfortable.”





