The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for MicroGEM’s Sal6830 SARS-CoV-2 Saliva Test.
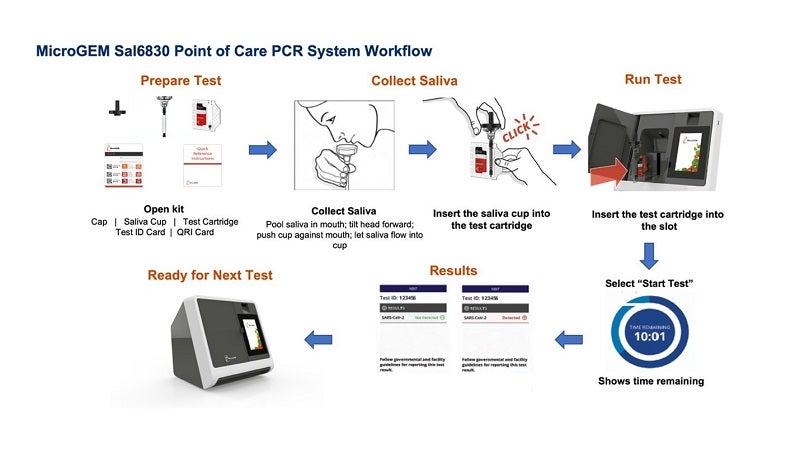
The test is claimed to be the nation’s first FDA approved saliva test for Covid-19 that uses polymerase chain reaction (PCR) directly at the point of care, providing results in 27 minutes.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
MicroGEM stated that the Sal6830 SARS-CoV-2 Saliva Test was clinically evaluated during the Delta and Omicron waves of the pandemic and has proven to be robust to viral mutations.
It has been designed for multiple SARS-CoV-2 gene targets, which enables robust detection of current variants and protects against obsolescence from future variants.
The MicroGEM Sal6830 SARS-CoV-2 Saliva Test’s simple workflow and easy saliva collection also reduce the workload for healthcare workers.
MicroGEM CEO Jeff Chapman said: “The MicroGEM Sal6830 SARS-CoV-2 Saliva Test will be an essential testing tool in our ongoing efforts to get our nation’s communities and businesses back to regular operations.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The introduction of the MicroGEM Sal6830 Point of Care PCR System marks a historic step in our mission to democratise molecular diagnostics by moving ultra-fast, high-performance testing out of laboratories and closer to people at the point of need, thus allowing decisions to be made in real-time.”
The company stated that the small size of the new saliva test allows it to be incorporated at testing sites that include ambulatory surgical centres, Clinical Laboratory Improvement Amendments (CLIA)-waived workplace testing sites, emergency departments and mobile testing labs.
The test received authorisation to detect nucleic acid from the SARS-CoV-2 virus only, and not for any other viruses or pathogens.





