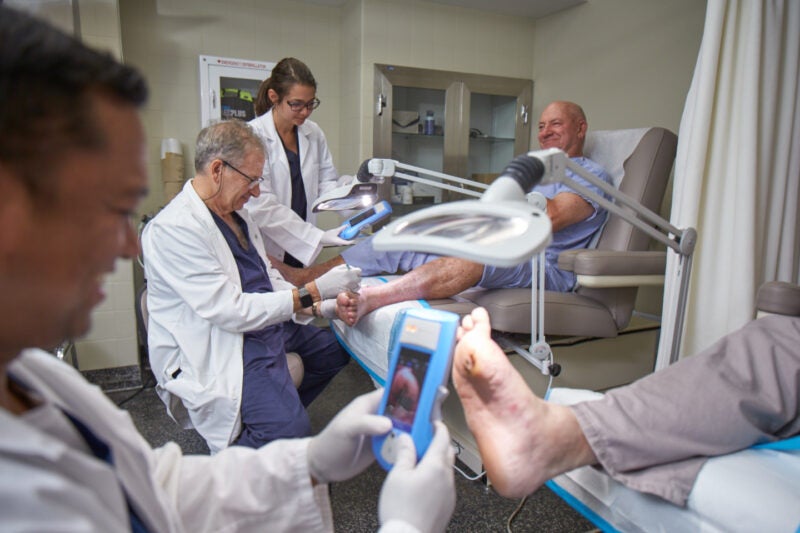
MolecuLight has reported that the use of its MolecuLight i:X device to view the presence of increased bacterial burden in wounds raised 12-week healing rates by 204% in a trial involving diabetic foot ulcer patients.
The randomised, independent, blinded, controlled trial enrolled 56 patients to assess the use of point-of-care bacterial autofluorescence imaging to manage diabetic foot ulcers versus the standard-of-care alone.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Patients were categorised into two groups based on MolecuLight device usage.
In one of the groups, the device was used twice a week to assess the presence of increased bacterial burden in diabetic foot ulcers.
In this MolecuLight arm, fluorescence imaging was carried out following treatment.
Fluorescence showed the presence of a high bacterial burden in more than 80% of the wounds.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the trial, no rise in the number of antibiotics prescribed was reported in the MolecuLight arm.
Apart from the two-fold improvement in healing rates, a link between baseline fluorescence and wound outcomes was observed in the trial.
In subjects with negative fluorescence images during the baseline visit, 53.9% were healed at 12 weeks compared to 37.5% with positive baseline fluorescence images.
Furthermore, if the wound was positive for high bacterial loads at the start of the treatment, as shown by MolecuLight, subjects were 36% more unlikely to have healed after 12 weeks.
The MolecuLight group had a superior reduction in the wound area as well as showing a deviation of participant quality of life toward improvement at four weeks. Meanwhile, the control arm deviated towards deterioration at 12 weeks.
The company noted that the trial validates the utility of the MolecuLight device in informing clinicians on clinically significant bacteria’s presence and location. It also enhances treatment plans and outcomes compared to standard diagnostic methods.
MolecuLight CEO Anil Amlani said: “A doubling of 12-week wound healing is a significant outcome and is consistent with what thousands of wound care clinicians are experiencing worldwide, that MolecuLight enables clinicians to deliver superior, proactive bacterial/infection management that improves wound outcomes.”
In June, the US Food and Drug Administration granted expanded 510(K) clearance for the MolecuLight i:X imaging device to identify wound regions containing elevated bacterial load as well as more bacterial species.





