Thermedical has received US Food and Drug Administration (FDA) approval for a clinical trial of its Saline Enhanced Radiofrequency (SERF) Ablation System.
The single-arm, open-label interventional clinical study has been designed to assess the efficacy and safety of the SERF Ablation System with Durablate Catheter in ventricular tachycardia (VT) patients who are resistant to antiarrhythmic drugs or standard ablation procedures.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
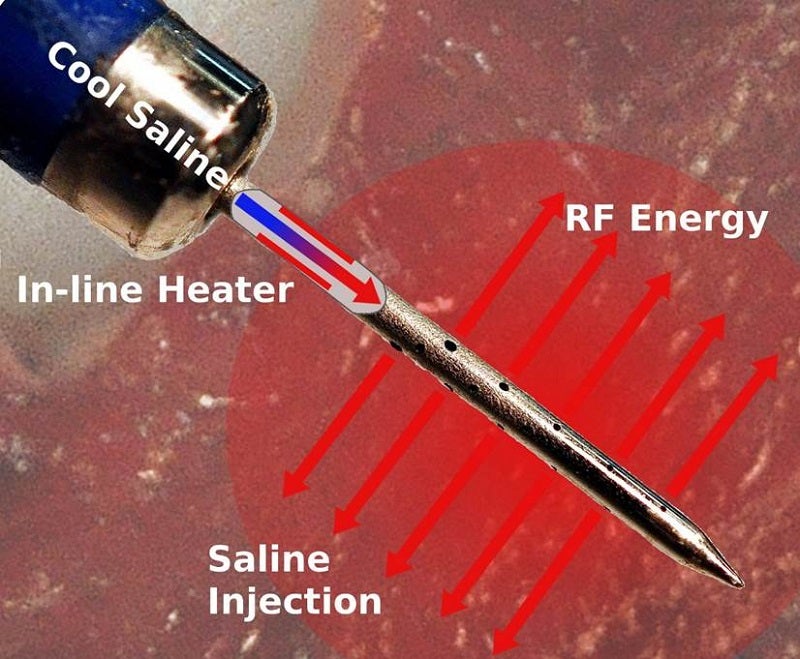
An electrosurgical generator, Thermedical Ablation System has been designed to provide RF energy while simultaneously injecting heated saline into a targeted location in the body for local thermal coagulation and soft tissue ablation.
It offers a new form of biological heat transfer and has been designed to be more efficient compared to conventional ablation approaches.
The Durablate catheter is intended to provide energy to control the ablation size and treat deeper heart wall tissues, where arrhythmias that cause VT are often located.
Thermedical co-founder and CEO Michael Curley said: “We are grateful to the FDA for the rapid approval of this pivotal trial to evaluate our Saline Enhanced Radiofrequency (SERF) Ablation System in patients who have run out of treatment options for their VT episodes and who suffer from extremely poor quality of life.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“In our recent multi-centre trial, 31 of 32 participants experienced immediate elimination of their clinical VT at the end of the procedure, and therapies such as shock or pace regulation were reduced by 89% during the five-month follow-up in these patients.”
The company noted that the Mayo Clinic in Rochester, Minnesota, will be the first of up to 25 investigational locations in North America to enrol participants in the clinical study.
Planned additional locations will include Philadelphia, Nashville, South Carolina, Alabama, Tennessee, Boston, Birmingham, Charleston, Montreal and Quebec City.
About 154 participants with recurrent, persistent, monomorphic VT who are resistant to drug therapy and conventional catheter ablation will be enrolled.
The participants in the clinical study will undergo a SERF ablation procedure and have follow-up visits at seven days, one month, three months and six months.
In 2020, Thermedical’s SERF ablation system and Durablate Catheter received Breakthrough Device Designation from the US FDA.





