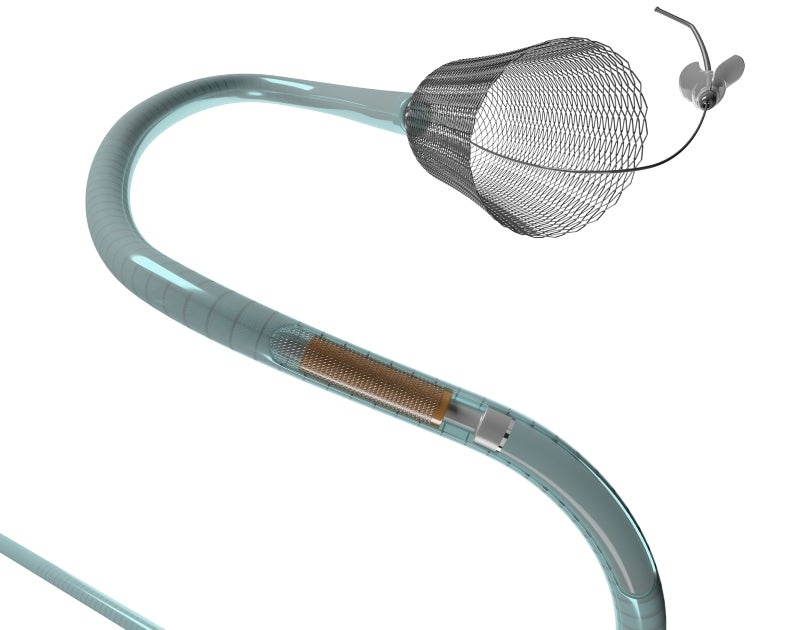
In February, Medtronic’s Pipeline Flex flow diversion device won expanded FDA approval to treat small or medium wide-neck aneurysms in addition to its existing approval for large or giant wide-neck aneurysms.
GlobalData estimates that the flow diversion stent market is growing at a Compound Annual Growth Rate (CAGR) of approximately 2%. Endovascular treatment for aneurysms is widely adopted as the go-to treatment, as it shows reduced complication rates and better patient outcomes compared to a surgical approach. However, the treatment of wide-neck aneurysms is associated with the risk of coil migration and aneurysm re-opening due to coil compaction. As such, support devices are often used to reduce these risks.
Flow diversion stents attempt to minimise the risks associated with treating wide-neck aneurysms. The stent is placed across the neck of the aneurysm and diverts blood flow past the aneurysm. Flow diversion stents can also be used in combination with coils, acting as both a support device to avoid coil migration and a flow diverter.
Since the Pipeline Flex has received expanded FDA approval to treat various sizes of wide-neck aneurysms, this will allow for more patients to be treated. GlobalData predicts that the inclusion of more indications will drive the growth of flow diversion stents in the near future. However, while this device has the potential to address some of the unmet needs of treating wide-neck aneurysms, it is still relatively new and will require more evidence from randomised clinical trials demonstrating minimal patient complications and significant results compared to alternative treatment devices.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData