Medtronic obtains FDA clearance for artificial pancreas system
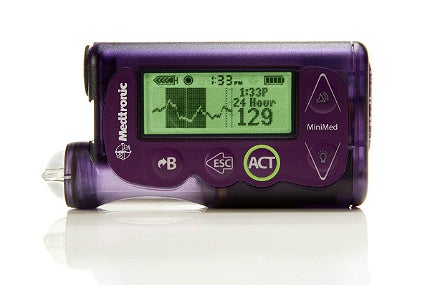
Medtronic has obtained US Food and Drug Administration (FDA) approval for its first generation artificial pancreas system, the MiniMed 530G with Enlite, with Threshold Suspend automation for diabetic patients.
It is reportedly the first system in the US capable of automatically halting insulin delivery when sensor glucose values fall below a predetermined threshold and when the patient fails to respond to the Threshold suspend alarm.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The MiniMed 530G system features the new Enlite sensor, Medtronic’s most accurate and comfortable continuous glucose sensor, with a 31% improvement in overall accuracy compared with the previous versions.
Stryker to acquire Mako Surgical for $1.65bn
US medical equipment manufacturer Stryker has signed a definitive agreement to acquire Mako Surgical, developer of robotic solution and implants for minimally invasive orthopaedic procedures, for $30 per share with an aggregate purchase price of approximately $1.65bn.
Mako Surgical markets joint-specific applications for the knee and hip, proprietary Restoris implants for orthopaedic procedures called MAKOplasty, and the Rio robotic arm interactive orthopaedic system.
The Rio is a surgeon-interactive tactile surgical platform that incorporates a robotic arm and patient-specific visualisation technology, which enables precise, consistently reproducible bone resection for the accurate insertion and alignment of Mako’s Restoris implants.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGiven Imaging secures Japanese clearance for PillCam SB system
Israel-based medical devices developer Given Imaging has received clearance from the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan for its third-generation video capsule PillCam SB3 to detect and monitor small bowel illnesses, such as Crohn’s disease.
The PillCam SB3 capsule is at the core of a minimally invasive procedure to visualise and monitor small bowel abnormalities associated with Crohn’s disease, iron deficiency anemia (IDA) and obscure GI bleeding (OGIB).
Weighing fewer than 4g, the 11mm by 26mm video capsule features an imaging device and a light source that can transmit anywhere between two and six images in a second.
Boston Scientific reports positive results from Lotus valve system study
US-based medical device manufacturer Boston Scientific has reported positive six-month results from the first 60 patients enroled in the REPRISE II clinical trial, designed to evaluate the safety and performance of the Lotus valve system in symptomatic patients with severe aortic stenosis, considered at high-risk for surgical valve replacement.
The Lotus transcatheter aortic valve replacement device with the company’s Adaptive seal is designed to minimise aortic regurgitation and is both fully repositionable and retrievable before release.
The data demonstrated excellent results with no new valve-related adverse events between 30 days and six months.
Olympus launches surgical tool for sinus and endoscopic skull base surgeries
US-based medical and surgical products designer Olympus has launched Diego Elite Multidebrider, a new tool designed to deliver optimal tissue dissection with built-in hemostasis and help speed-up minimally invasive sinus surgeries.
The Diego Elite Multidebrider surgical tool can be used in polypectomy, tonsillectomy, adenoidectomy and a number of laryngeal surgeries, among other procedures.
It is reportedly the only ENT system that offers monopolar and bipolar energy blades for hemostasis, as well as traditional standard shaver blades, reducing the need for other instruments.
Ventana and Boehringer Ingelheim to develop companion diagnostic tests
Ventana Medical Systems (Ventana), a member of the Roche Group, has signed a collaboration agreement with German pharmaceutical group Boehringer Ingelheim to develop companion diagnostic tests for Boehringer’s oncology programmes.
Companion diagnostics are assays or tests that are intended to assist physicians in making treatment decisions for their patients based on the best response to therapy.
Under the agreement, Ventana will provide access to its immunohistochemistry technology platform, experience and expertise in developing companion diagnostics and premarket approval (PMA) submissions to Boehringer Ingelheim.
Precision Spine launches new anterior cervical plate system in US
Precision Spine, a US-based spinal device company, has launched its Slimplicity solo anterior cervical plate system, which recently received 510(k) clearance from the US Food and Drug Administration.
Slimplicity solo is designed for anterior cervical fixation to the cervical spine C2-C7 for indications including degenerative disc disease, trauma (including fractures), tumours, deformity (such as kyphosis or scoliosis), pseudarthrosis, failed previous fusion, spondylolisthesis and spinal stenosis.
The new anterior cervical plate system, which consists of one and two level plates and 4.5mm fixed and variable screws, is designed to accommodate a visual locking confirmation with a secure locking mechanism, and provides an ergonomic colour-coded instrument set.
Medtronic reports positive preliminary results from Global SYMPLICITY Registry
Medtronic has reported preliminary information from the Global SYMPLICITY Registry, a multicentre, prospective, observational registry designed to collect comprehensive data evaluating procedural and long-term safety of the Symplicity system.
The main objective of the Global SYMPLICITY Registry is to confirm procedural safety with the Symplicity system.
There were no major complications or serious adverse events related to delivery of radio frequency (RF) energy to the renal artery other than one procedural dissection (0.09%) and one re-intervention at six months (0.09%) in 1,158 patients analysed in the registry with available follow-up information.