Despite being one of the most commonly studied psychiatric disorders in children, uncertainty and controversy still surround diagnosing attention deficit hyperactivity disorder (ADHD).
The common chronic disorder is said to affect 3%-5% of children worldwide, with many showing symptoms before the age of seven. It is believed 30%-50% of those individuals then carry the symptoms into adulthood.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
ADHD-related behavioural problems
Be it at home, in school or through their relationships with peers, children with ADHD can suffer from a range of behavioural problems due to a short attention span. If untreated, the disorder is said to have long-term adverse effects into adolescence and adulthood. The core symptoms of ADHD are impulsiveness, hyperactivity and inattention.
In 2008, US diagnostics systems company BioBehavioral Diagnositcs (BioBDx) launched the first Federal Drug Administration (FDA)-cleared ADHD testing device aimed at providing healthcare professionals with an office-based device that measured the three core symptoms of ADHD.
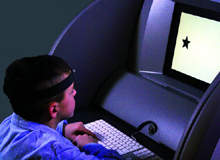
Quotient ADHD system

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Quotient ADHD system uses a motion-tracking system to measure an individual's movement while he or she attempts to focus on changing visual stimuli. Special software then analyses the motions and compiles a report on its accuracy, which qualified professionals will use to help identify ADHD symptoms.
Although the device is relatively new to the US market, BioBDx's vice-president of marketing, Carrie Mulherin, says its research and development has been ongoing for many years now.
"The product was actually cleared by the FDA in 2002 and its primary research dates all the way back to publications from the mid-1990s, which look at motion tracking and attention space analysis. This approach to ADHD was very different and a number of product enhancements were needed in order to make it commercially viable," she says.
"Over a six-month period, the software was trialled on 220 people, whose metrics and genders were all matched and compared. We also had hardware issues to address too, basic things like the colour and shape of the stool."
There are four basic components of the Quotient ADHD System. The motion-tracking system captures each movement 50 times a second and plots the pattern of movement. The reflectors are used to monitor pattern movements – these are placed on the forehead of the patient, and on the shins if the patient is under 13 years old. The LCD screen randomly flashes geometric shapes the patient must respond to, and, lastly, the keyboard is used by the patient to respond to visual stimuli.
The components are manufactured by the US-based medical and clinical device manufacturer Cogmedix, which signed a contract with BioBDx in May 2009. The company's president and CEO Chris Coghlin says he believes the production rates for the Quotient system will increase rapidly in the coming years.
ADHD diagnosis
BioBDx claims that the results compiled by the Quotient ADHD system's software are over 90% accurate in identifying ADHD. The software, which calculates results based on 19 or more parameters, produces a four-sectioned report identifying: motion analysis, attention analysis, shift in attention state and Quotient composite scores. The report can then compare the results of a boy with ADHD symptoms with a boy in the same age category without the symptoms.
The Quotient ADHD test takes 15 minutes to complete for children up to the age of 13, or 20 minutes for adolescents and adults. The report is then made available to the clinician in under a minute, who uses the results in conjunction with other assessment tools and a clinical exam to form a treatment plan for the patient.
"The biggest benefit of the Quotient ADHD test is that it offers a real-time picture of the neo-processes of the brain that is executing the test. In contrast with other ADHD methods that predominantly involve rating scales and subjective questionnaires with teachers, parents and the patient, the system offers an incredibly objective approach," Mulherin says.
"The Quotient ADHD system also has the ability to measure other mental disease states that rely on attention and motion tracking, so for example we are holding clinical trials for anxiety and depression. Parkinson's is a condition we may look into in the future and we have also been talking to doctors about working with concussion and head injury cases. There are a number of possible expansions within the current platform we have now."
To date, approximately 60 devices are in use across the US, predominantly on the east coast. BioBDx has recently expanded its domestic sales team in order to achieve a more widespread adoption of the technology before it begins marketing the product in Europe.
"Right now we are mainly targeting the Quotient ADHD system at psychiatrists, neurologists and some clinicians. We haven't figured out yet how the system will evolve within a school environment," Mulherin says.
"The results will eventually speak for themselves, but at the moment it's a case of establishing best practices for the system in the US and then in Europe."





