
While GLP-1 receptor agonists (GLP-1RAs) are taking up attention in the weight loss market, steady, incremental progress is quietly unfolding in the non-pharmaceutical realm of medical foods.
Stockholm based company Sigrid Therapeutics is pioneering Carb Fence, a medical food designed for blood sugar and weight management in prediabetes, type 2 diabetes, and obesity.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Sigrid has secured US market access for the medical grade product off the back of results from its SHINE study in prediabetes (NCT06087822). The trial found Carb Fence, reduced long-term blood sugar levels HbA1c, improved glucose metabolism, and reduced body weight – while preserving muscle mass. Medical food is a category recognised by the US Food & Drug Administration (FDA) for the dietary management of diseases or conditions under physician supervision, as defined by the Orphan Drug Act.
Carb Fence utilises SiPore, a technology based on mesoporous silica particle (MSP) – a colloidal amorphous silicon dioxide matrix that functions like a molecular sieve. The technology aims to prevent the interaction between digestive enzymes and food, inhibiting the breakdown of food into particles that the body can absorb.
Due to this method of action, the company claims that Carb Fence can transform traditional fast food into what it calls ‘slow food’ – negating the direct glucose impact of high fat and sugar foods.
The SHINE trial sought to assess the safety and tolerability of SiPore-powered Carb Fence while confirming its efficacy across key metabolic markers. Recruiting 318 participants onto the trial, researchers found Carb Fence significantly reduced HbA1C levels from baseline in both males and females, with the female group achieving a clinically relevant, statistically significant, placebo-adjusted reduction.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn a presentation to investors, the company shared that the SHINE trial was able to establish Carb Fence’s ability to induce a 0.9 mmol/mol reduction within three months, compared with Metformin’s 0.06 mmol/mol after six months.
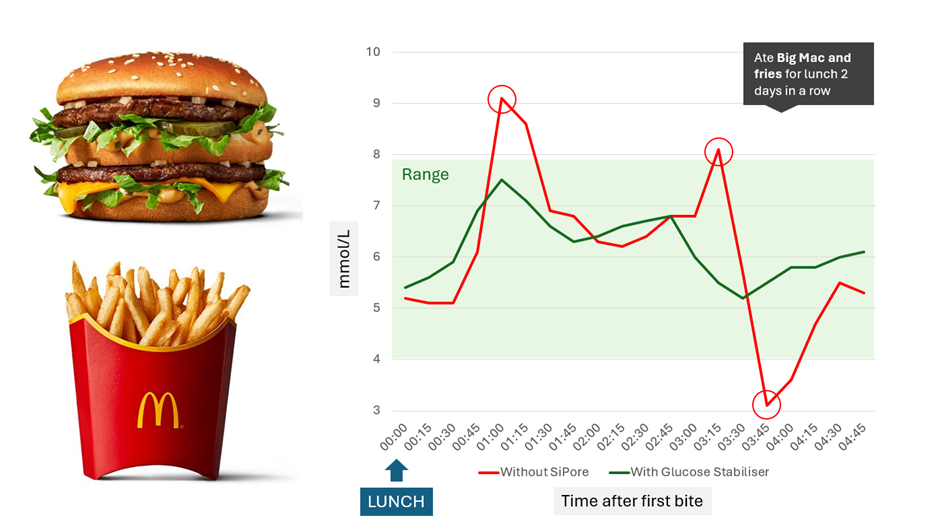
According to Sigrid, feedback from users in the SHINE trial has been positive. The company shared anecdotal data it received from a single trial participant who used their continuous glucose monitor (CGM) to assess the HbA1c impact of a McDonalds Big Mac meal (without a drink). That data found that the user achieved more stable blood sugar after consuming the Big Mac meal whilst using Carb Fence.
Following the recognition, the company has now set its sights on approval in the EU before the Europeans Medicines (EMA) agency. The company has also secured $4m in a funding round to advance the development and commercialisation of its SiPore technology.
Speaking with Medical Device Network, co-founder & CEO of Sigrid Therapeutics, Sana Alajmovic, detailed how it is looking to take a piece of the market left behind by traditional GLP1 therapies.
Joshua Silverwood: Tell me a bit about how Sigrid got here
Sana Alajmovic: We now have four studies under our belt that confirm the safety, and the efficacy of carb fence and SHINE was our pivotal clinical study, one of the largest of its kind within pre diabetics where we successfully demonstrated that we can lower long term blood sugar, which was our primary endpoint.
It is faster and more effective than metformin which is currently used off label to manage blood sugar levels in pre-diabetics. Our future plan is to take on the US first due to the fact we were able to secure clearance as a medical food to be used to under the supervision of a physician for the dietary management for disease. In our case obesity and prediabetes.
The plan now is to work with clinics and key opinion leaders to start using Carb Fence with their patients. We are first looking to target GLP1 patients who are looking to transition off their treatment.
JS: It seems like you are targeting the same market as drugs like Ozempic, can you tell me about that?
SA: We recently had a big shareholder presentation and of course, the main question is always whether we know who we are targeting. So, we are targeting four groups of people, with this product. We believe that this can become a first-line treatment for pre-diabetes. There are more than half a billion people who are living with prediabetes today. There are many people who don’t know it, but their awareness is increasing. That’s the precursor stage of type-2 diabetes, and right now there are no approved agents for blood sugar control in prediabetics and we hope to become the first one here.
We also have type 2 diabetics, we see this as a potential adjunct therapy as we have done drug, and device interaction studies with Carb Fence and we’ve seen that it is perfectly fine to combine these two. Thirdly, there are a lot of people who can’t tolerate GLP1 medications and don’t have access to any kind of alternative. Lastly, there are those for whom GLP1 therapy is just still too expensive for them.
Here we believe we can be a more gradual and sustainable weight loss treatment. We don’t have the drastic efficacy of GLP1 drugs, but this is a more sustainable long-term treatment to lose weight. So, we have a role to play there.
We are also targeting those looking to maintain their newfound body weight for people transitioning away from GLP1 treatments. 85% of people on GLP1 therapies will quit after two years. Once you stop taking these injections you are likely to regain almost two-thirds of the weight that was lost, so people will go back and forth on GLP1s, so if you can give them something that can stop this weight gain, I think we could be very successful there.
JS: The regimens for treatments such as Ozempic can be intensive, is this more about targeting people who would be adverse to that?
SA: Absolutely, there are still a lot of people who don’t like injections. This is an oral solution. You drink it, it’s a medical grade fast-acting liquid, with a mild peach flavour, that for us has had very high patient satisfaction. I think the downside then is that you have to take this every day in case you want to have these strong healthcare benefits. Whereas with GLP1 you only have an injection once a week, but if it’s tasty enough, you also want to take it.
JS: You talked about catering to patients tapering off GLP1 drugs, have you conducted any studies examining patients tapering off of Carb Fence?
SA: We have not, we don’t have that data. We only have data for three months. What we do have is a glucose dietary supplement that has been on the market for a while, here we can see that a lot of people are using it to maintain their weight. We don’t believe so, but we have not done the studies to prove it.
JS: Can you elaborate on this claim of turning ‘fast food into slow food.’ Could I eat a fast-food meal and effectively have it not count?
SA: One of our users wanted to test the product to see how good it was, he lives in the States and uses a CGM, he went to McDonalds and ate a Big Mac and fries. Without Carb Fence you can see that his blood sugar has spiked quite drastically, outside of the targeting range of his CGM. Once he took the capsule the next day did the same thing, and you can see that his blood sugar was kept much steadier. His blood sugars were within the right time and range. It was within a healthy level. These are the types of reviews we have been getting from patients.





