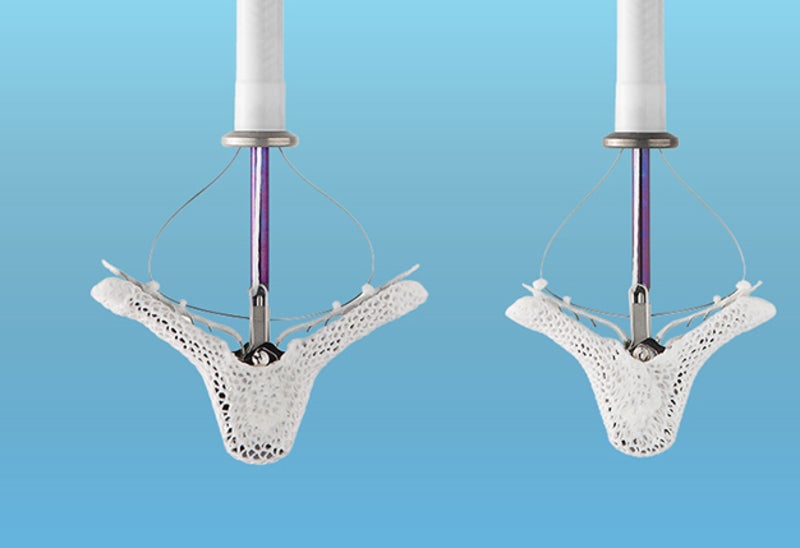
The US Food and Drug Administration (FDA) has expanded the indication of Abbott’s MitraClip device to include the treatment of heart failure patients with clinically significant secondary or functional mitral regurgitation (MR).
In July last year, the regulatory authority approved the device for repairing leaky mitral valves without open-heart surgery.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Abbott structural heart business chief medical officer Neil Moat said: “Since severe secondary MR is extremely difficult to manage and associated with a poor prognosis, people have historically had few options.
“The expanded indication of MitraClip opens new doors for these ailing patients and can improve their quality of life and chance of survival despite their complex condition.”
The MitraClip device joins parts of the mitral valve together to mitigate backflow of blood and restore the heart’s ability to pump oxygenated blood more efficiently.
The FDA’s approval is based on results from the COAPT Trial, which evaluated MitraClip for the treatment of secondary MR. The trial met both primary and all ten secondary endpoints.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDuring the trial, a 47% relative decrease in hospitalisations and a 38% relative reduction in mortality were observed, demonstrating benefits to medical therapy.
A total of 614 symptomatic heart failure patients with moderate-to-severe secondary MR participated in the trail, which was conducted at 78 centres in the US and Canada.
The primary efficacy endpoint of the study was a reduction in heart failure hospitalisations in two years, while the primary safety endpoint was freedom from device-related complications at one year compared to an 88% performance goal.





