Aerin Medical, specialists in developing treatments for chronic nasal conditions, has published positive two-year follow-up data from the AERWAY trial for patients with severe or extreme nasal airway obstruction (NAO) due to nasal valve collapse (NVC).
The results, featured in Laryngoscope Investigative Otolaryngology, showed a significant and long-term improvement in NAO symptom burden.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The prevalence of nasal airway obstruction in the US is approximately 30%. Of the patients seeking treatment, an estimated 63% have severe or extreme nasal airway obstruction.
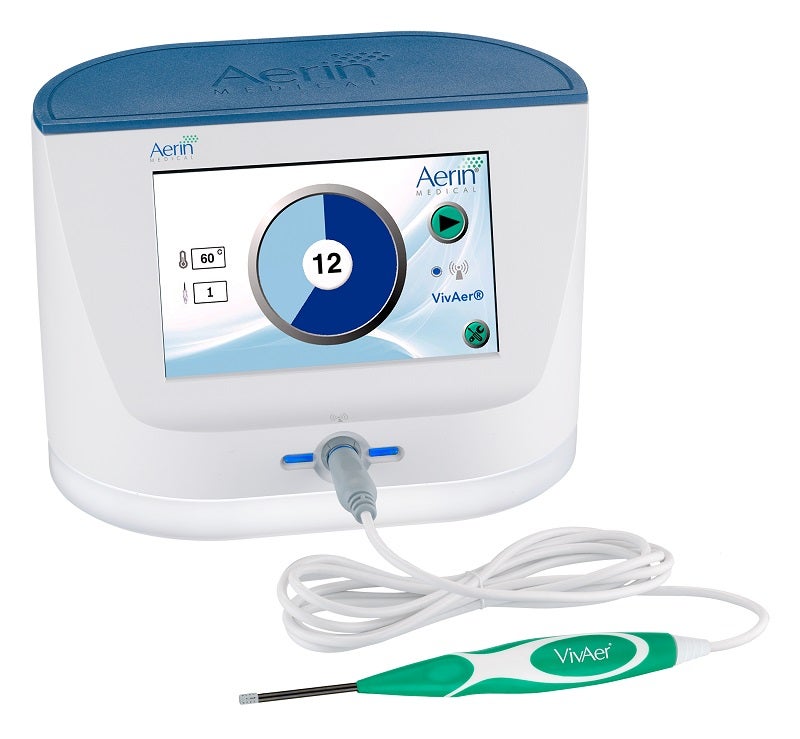
VivAer is a small stylus placed into a patient’s nostril to precisely target and treat sources of nasal blockage such as nasal valve collapse and other soft tissues causing obstruction in the nose.
The wand-like stylus uses low-temperature radiofrequency energy to gently remodel the nasal passage and improve airflow. Treatment is minimally invasive, and patients report minimal discomfort with little downtime following the procedure.
The AERWAY study (NCT04277507), conducted at 12 clinical study sites across the United States, demonstrated that the VivAer is safe and effective. Patients sustained two-year symptom improvement consistent with three-month, six-month and one-year results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe study’s findings showed a statistically significant and sustained improvement in symptom burden in all subpopulations including NVC patients with untreated septal deviations and either static or dynamic NVC.
Moreover, 90.1% of the patients showed improvements in total Nasal Obstruction Symptom Evaluation (NOSE) Scale scores, which were maintained for two years.
The study saw a decreased use of oral medications, nasal sprays and breathing strips at two years compared to baseline, and treatment was well-tolerated, with no serious adverse events related to the procedure or the device.
The study also showed similar significant nasal obstruction symptom relief in patients with and without septal deviations, following a single in-office treatment with VivAer. Although VivAer does not directly treat a deviated septum, it can help improve airflow when a nasal valve collapses by gently remodelling the lateral nasal wall from the inside of the nostril, creating more space for air to flow.
Furthermore, the VivAer procedure could be considered as an initial option prior to pursuing a surgical intervention.
“Over 90% of patients in the study had sustained statistically significant improvements in symptoms of nasal airway obstruction at two years. This is important to show that the treatment effects from VivAer are durable over time,” said Desiree Hollemon, MSN, MPH, Vice President of clinical affairs with Aerin Medical.
Aerin also reported earlier positive results for VivAer in June 2022.





