Akura Medical, a Shifamed portfolio company, has closed its Series B financing round, through which it raised $35m in funding.
The funds will be used to apply for US Food and Drug Administration (FDA) 510(k) approval for its mechanical thrombectomy platform.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Led by the Capital Partnership (TCP) and Cormorant Asset Management, the financing round saw participation from the PA MedTech VC Fund, Lilly Asia Ventures, AMED Ventures, Unorthodox Ventures and Shifamed angel investors.
The Capital Partnership managing director Gautam Kainth said: “Despite the proliferation of mechanical thrombectomy devices, significant challenges remain. The low-profile Akura platform is designed to remove soft and hard clots.”
Besides utilising the funds to apply for 510(k) clearance from the FDA for the thrombectomy platform, Akura intends to use the proceeds to carry out clinical studies for additional indications for the platform and scaling manufacturing capabilities.
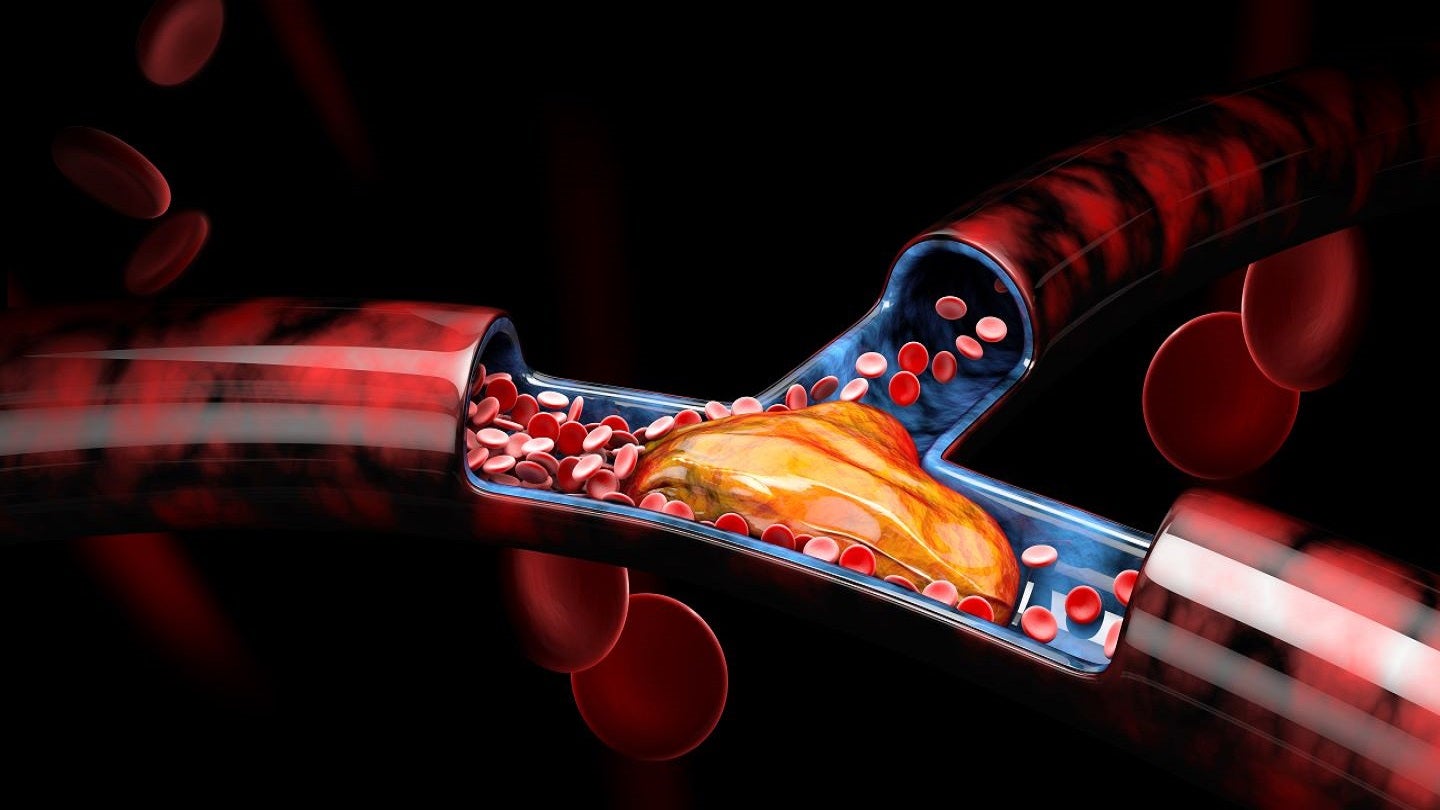
Designed to be an easy-to-use, smaller bore system, the Akura mechanical thrombectomy platform is a low-profile, steerable sheath that is said to help physicians bring the catheter tip directly to the proximal end of the clot without requiring to cross the clot.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIncorporating intersecting jets, the platform’s integrated aspiration and maceration technology facilitates the effective removal of mixed morphology clots.
Furthermore, its pressure sensors deliver real-time hemodynamic data, thereby cutting down the guesswork around case progress.
This mechanical thrombectomy platform, which is currently in the research and development phase, has not yet received CE mark approval. It is also not approved for sale in any part of the world.
Akura Medical president and CEO Murali Srivathsa said: “The Akura thrombectomy platform is designed to provide large-bore catheter performance with a user-friendly system that will change how physicians approach thrombus removal in a clinically meaningful way.
“We thank our investors for their strong, ongoing support as we continue to execute and deliver on our commitment to advance our differentiated solution for VTE [venous thromboembolism].”





