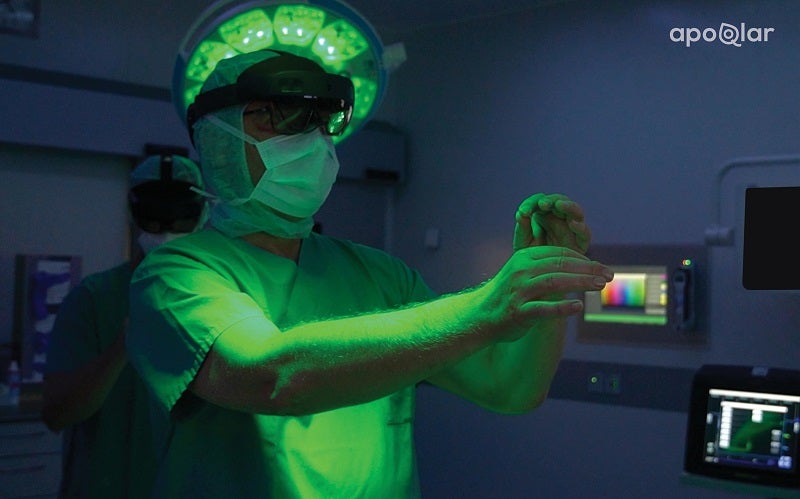
German medical metaverse company apoQlar has secured 510(k) Class II clearance for its mixed reality surgical planning platform, VSI HoloMedicine, from the US Food and Drug Administration (FDA).
With the new software device, surgeons will be able to use immersive 3D holographic technology to plan for complex surgeries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
VSI HoloMedicine uses the technology to provide surgeons with what is described as similar to x-ray vision for surgical planning processes.
It brings a third dimension to surgical planning, patient education, remote consultation and medical training.
Doctors from all medical specialities can use it to visualise medical data inside or outside the operating room and plan surgeries in 3D.
Surgeons can transform flat computerised tomography (CT), angio CT, magnetic resonance imaging (MRI), cone-beam CT (CBCT), positron emission tomography (PET) and single-photon emission CT (SPECT) sources into interactive 3D holograms using Microsoft’s HoloLens 2, which is a mixed reality-based head-mounted display (HMD).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataapoQlar co-founder and CEO Sirko Pelzl said: “With mixed reality, we are no longer bound to physical objects in a physical world. We can leverage digital objects and services on top of the real world for equal or greater utility and usually at a fraction of the cost.
“Mixed Reality is a completely new way for people, and in our case surgeons, physicians and technologists, to continue to experience the real world around them but with an entire virtual layer placed on top.”
The company stated that the US is the 30th nation to provide medical certification for the system.
The device has already received FDA 510(k), CE Class I as well as Health Sciences Authority (HSA) Class A medical certifications for clinical use in 30 countries.
Slated to be available in the second quarter of next year, apoQlar intends to achieve VSI HoloMedicine’s distribution for clinical use in the US through its subsidiary in Miami, Florida.
At present, the company is conducting its first-ever funding effort to raise a Series A round that would support scaling VSI Holomedicine as the foundation of modern surgical care.





