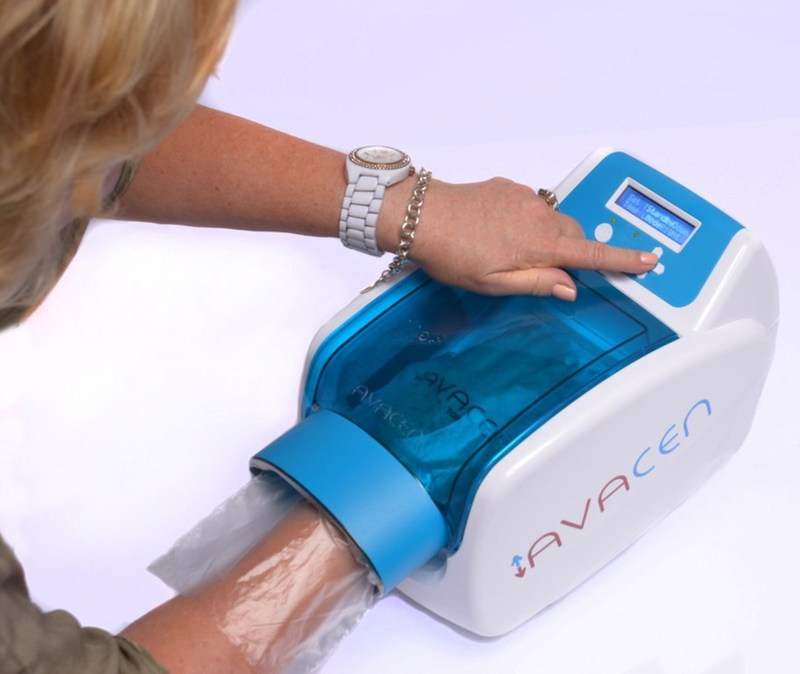
Avacen Medical is set to investigate its US Food and Drug Administration (FDA) cleared AVACEN 100 device in a clinical trial for the treatment of autism behaviour.
AVACEN 100 is an over-the-counter, non-invasive medical device that is currently indicated to provide temporary relief for arthritis, muscle and joint pain.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The firm intends to submit the results from the double-blind, randomised, placebo-controlled study to the FDA for approval to treat social interaction and communication deficits, as well as for minimising anxiety level in autistic children and adolescents.
Planned for the first quarter of this year, the clinical study will assess the device in patients aged 6-16 years from military families in the San Diego region of the US.
Avacen Medical CEO Thomas Muehlbauer said: “We will be donating an AVACEN 100 to each family completing the study.
“If the child does not respond, our device can still offer benefits for each family member or their grandparents.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Although I admit there is geographical and cultural value inherent in using military subjects, we feel there is just as much value associated with helping the San Diego military community.”
According to the firm, children who used the medical device for 15-30 minutes one or two times per day experienced relief from autistic behaviour with improvements in speech, classroom participation and focus.
AVACEN 100 currently holds patents in the US, China, Australia, Japan, UK, France, Germany, Spain, Sweden, and Canada.





