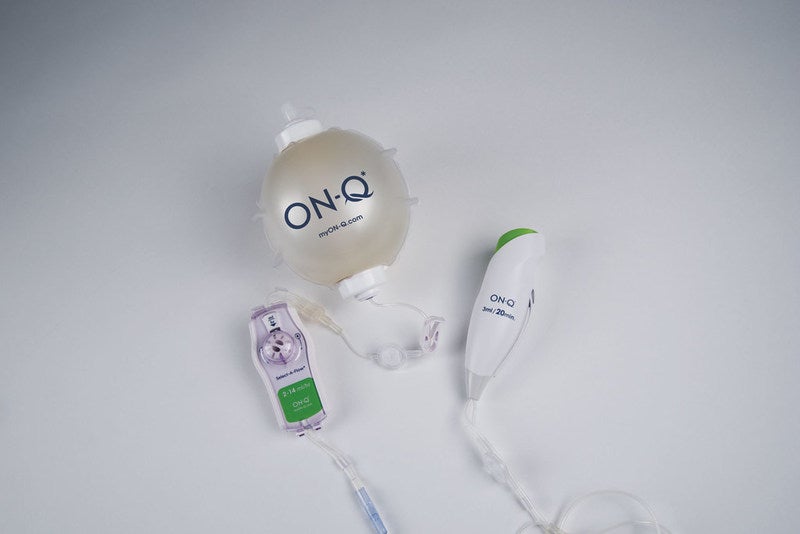
Avanos Medical has secured US Food and Drug Administration (FDA) 501(k) clearance for its postoperative pain management device ON-Q Bolus.
The device is designed to reduce the US’s reliance on opioids, offering an easy-to-use alternative for patients that are recovering from post-surgical pain.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to studies, the administration of discrete amounts of medication (as low as 3ml) in addition to the basal rate can cut the total amount of anaesthetic required for pain relief.
ON-Q Bolus is intended to offer improved pain relief compared to opioids alone.
The device is based on the ON-Q pump, which automatically delivers a regulated flow of local anaesthetics to the surgical site or peripheral nerves. The new device offers customised control for patients for up to five days. It features an easy-to-remove priming tab and large level indicator markings.
Avanos Medical CEO Joe Woody said: “The changes we have made to the ON-Q Bolus pump are a direct result of the feedback we heard from our customers.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Non-opioid approaches to pain management continue to be an important issue in the healthcare industry, particularly in light of the opioid crisis.
“As a leader in non-opioid treatment for acute pain, we are committed to continuously improving our ON-Q pumps to deliver increased quality of life and satisfaction to all patients.”
The pump will be commercially launched in the near future.
Avanos Medical’s portfolio features other pain pumps, including the ON-Q TRAC and ON-Q T-bloc Trays.



