Amplitude Vascular Systems (AVS) has signed an agreement with the Jacobs Institute in New York, US, to conduct clinical studies of the Pulse intravascular lithotripsy (IVL) system.
A nonprofit medical device innovation centre, Jacobs Institute is led by Dr Adnan Siddiqui.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The collaboration aims to assess the Pulse IVL system in both coronary and carotid vasculatures and intends to expand its clinical indications.
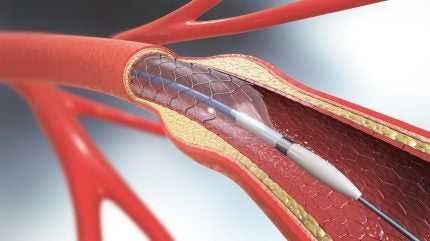
The Pulse IVL system utilises high-frequency pressure waves to fracture calcium within arterial walls, using a non-compliant balloon to facilitate vessel expansion.
Siddiqui said: “By fostering an environment that nurtures innovation, the JI will help to bring this technology to patients sooner, ultimately providing better treatment options for patients with coronary and carotid artery diseases.
“We are especially excited about studying the Pulse IVL system in carotids to improve stroke care. We believe its unique mechanism of action may be an important solution for the diffuse and eccentric nature of many of our carotid disease patients.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe partnership is set to leverage the Jacobs Institute’s clinical and regulatory expertise to potentially accelerate the market introduction of the Pulse IVL System for coronary and carotid applications.
AVS board chairman Mark Toland said: “Dr Siddiqui and the team at the JI are committed to bringing novel technologies to market through their vertically aligned infrastructure, which accelerates the pathway from development to clinical trials to commercialisation. We are excited to partner with the JI on expanding the use of the Pulse IVL system in coronary and carotid lesions.”
In June 2024, the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) to a pivotal trial investigating AVS’s pulsatile intravascular lithotripsy therapy.
The POWER-PAD-II clinical study will evaluate the safety and efficacy of AVS’s Pulse IVL system for the treatment of patients with severely calcified peripheral arterial disease.
The trial is anticipated to begin enrolment shortly at up to 20 US centres. Patients will be followed for up to six months after the therapy.





