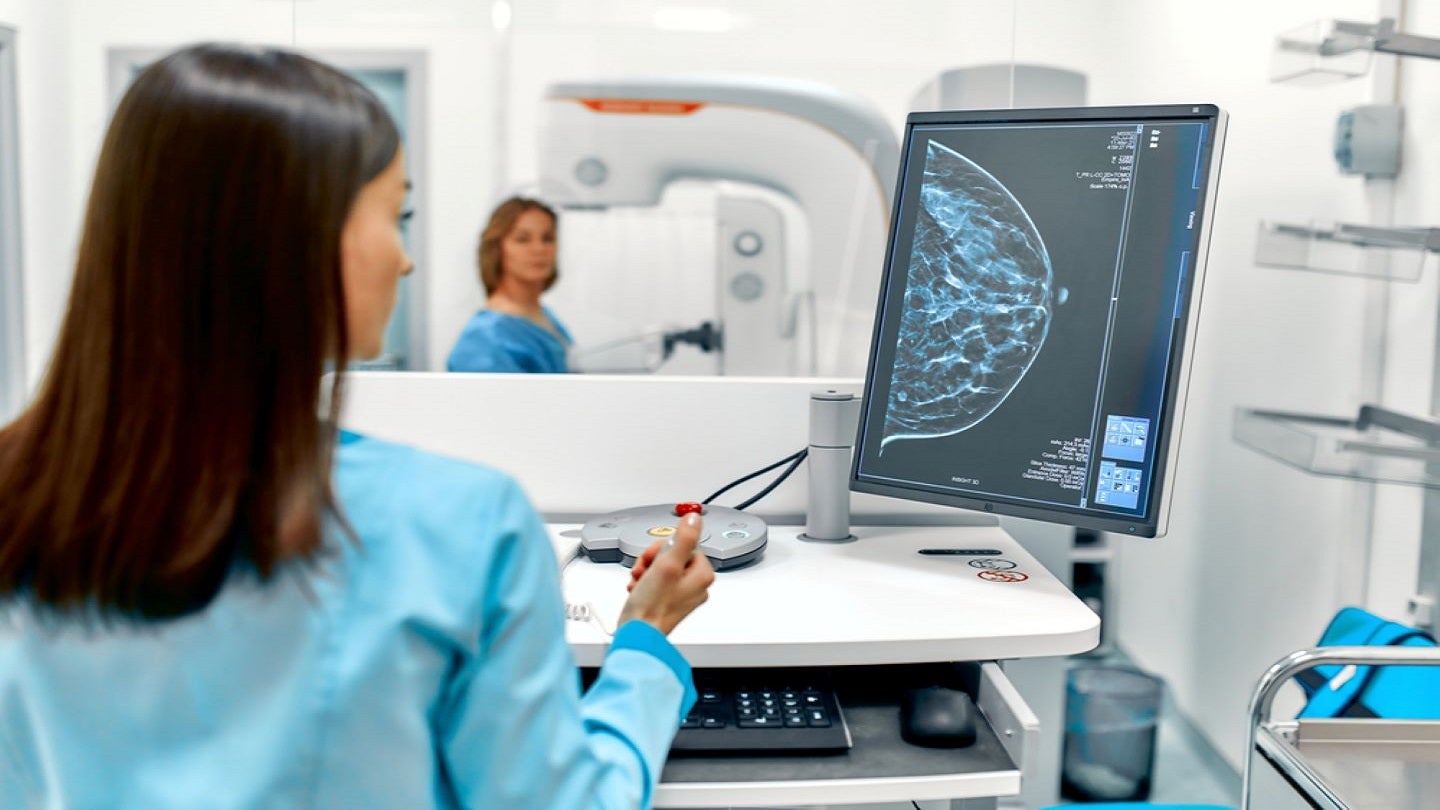
The US Food and Drug Administration has granted clearance for Bayer’s iodine-based agent, Ultravist(iopromide)-300 and 370, for contrast-enhanced mammography (CEM).
Ultravist is intended for the visualisation of suspected or known lesions of the breast in adults, as an adjunct to ultrasound or mammography.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
CEM combines digital mammography with the administration of a contrast agent like Ultravist to facilitate the identification of breast lesions.
Ultravist can be used in intra‐arterial procedures, including radiographic assessment of cardiac chambers and related arteries in paediatric patients aged two years and older.
It is also suitable for use in intravenous procedures, including excretory urography in adults and paediatric patients aged two years and above.
Bayer Radiology research and development head Dr Konstanze Diefenbach said: “The approval of Ultravist-300 and 370 in contrast-enhanced mammography gives physicians a new imaging option where conventional mammography might not be enough.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We are pleased to be able to offer additional options for breast imaging to healthcare professionals, as we aim to support them in their role of providing clear answers from diagnosis to care for patients.”
Bayer’s breast imaging portfolio also consists of a gadolinium-based contrast agent, Gadavist (gadobutrol), which was approved for use with MRI to evaluate the presence and extent of malignant breast disease in adults.
The company’s MEDRADStellant FLEX Computed Tomography Injection System with CertegraWorkstation was also approved in the US in 2019 for use in CEM.





