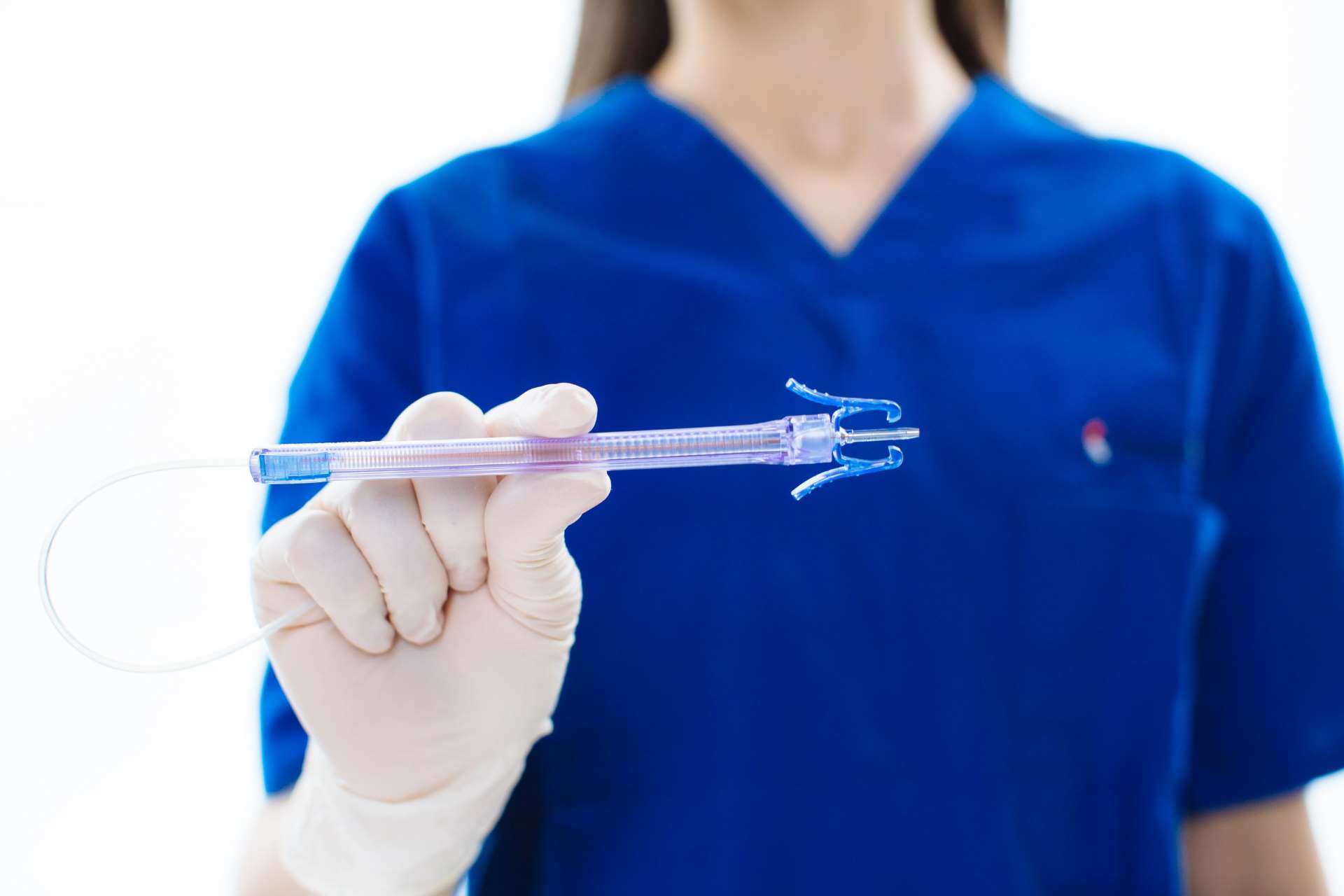
Becton, Dickinson and Company (BD) plans to introduce a ‘One-Stick Hospital Stay’ approach by leveraging its expertise in blood collection and vascular access solutions.
The company will use the needle-free technology of Velano Vascular, which it recently acquired for an undisclosed amount.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Velano’s technology facilitates blood draws from current peripheral intravenous catheter (PIVC) lines, avoiding numerous needlesticks for collecting blood samples.
This approach can lower the pain and discomfort linked with the process, which in turn could lead to improved outcomes and enhanced satisfaction for patients.
The Velano Vascular acquisition also included its US Food and Drug Administration (FDA)-approved PIVO device, which is a needle-free vascular access device that could enhance patients’ blood draw experiences.
This device will aid in BD’s ‘One-Stick Hospital Stay’ goal, where some low-acuity patients will only experience one needlestick throughout their stay in the hospital.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBD medication delivery solutions president Rick Byrd said: “As a global leader in vascular access solutions and blood collection, BD has been on the forefront of innovations that improve the patient experience and reduce additional needle sticks.
“Now, with the addition of Velano and its groundbreaking technologies, we can envision a world where a ‘One-Stick Hospital Stay’ could be a reality for many by making the numerous inpatient blood draws better for everyone.”
BD’s Vacutainer product portfolio enables safe and comfortable blood sample collection, the company said.
Furthermore, BD’s vascular access management solutions aid doctors in selecting the correct device for patients, enabling ‘first-stick success’ for beginning intravenous (IV) lines while lowering IV complications and expanding catheter dwell duration.
Currently, blood draws using venipuncture and inserting PIVCs are two frequent hospital procedures.
Needlesticks cause discomfort for nearly every patient hospitalised and, when used successively, are related to anxiety and some pain.
Estimates show that nearly 90% of hospitalised patients need IV therapy, with 95% of these devices being PIVCs.
Together, BD and Velano Vascular intend to address these issues and work towards the ‘One-Stick Hospital Stay’ goal.
Last month, the FDA expanded 510(k) clearance for BD’s PeritX Peritoneal Catheter System to drain symptomatic, recurrent and non-malignant ascites.





