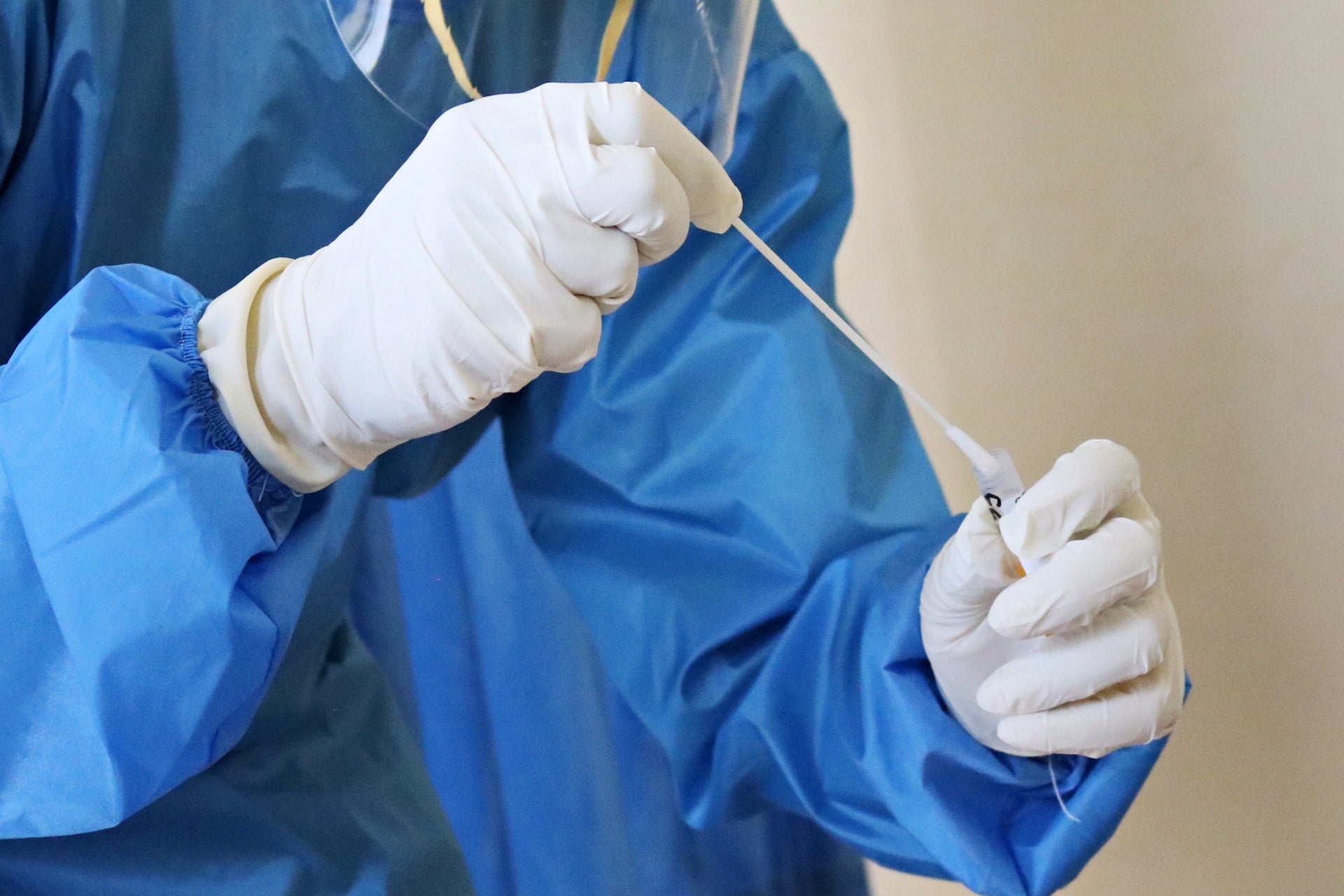
Biocept has introduced a new single test that can identify and differentiate between SARS-CoV-2 and influenza, allowing patients and healthcare providers to adopt the most suitable therapies.
The test adds to the company’s Covid-19 testing programme, which was initiated in June last year and has processed more than 670,000 samples.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It leverages a sensitive and precise reverse transcription-polymerase chain reaction (RT-PCR) platform to distinguish between Covid-19 and influenza.
With samples obtained using nasal swabs, which are processed in the company’s CLIA-certified and CAP-accredited laboratories, the combined Covid-19 and influenza test can deliver results within roughly 48 hours from receiving a specimen.
The company is currently extending the Covid-19 testing services that it conducts using the US Food and Drug Administration (FDA)-cleared diagnostic platform and kits of Thermo Fisher Scientific.
Biocept president and CEO Michael Nall said: “As we navigate through a complicated flu season, expanding our Covid-19 testing services to include influenza testing allows us to better meet the needs of our customers and our communities.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Because of the similarities in symptoms, determining whether a patient has Covid-19 or the seasonal flu can help patients and physicians make decisions about care that may lead to reduced viral spread and more efficient utilisation of healthcare resources.
“This new offering demonstrates our continued effort to support public health initiatives and provide customers with the answers they need.”
Meanwhile, the marketing of the Covid-19 assay jointly developed by Biocept and Aegea Biotechnologies will be postponed due to recent modifications in the FDA requirements for Covid-19 tests developed in laboratories.
In March, the companies entered a supply agreement for a PCR-based Covid-19 assay kit that leverages Switch-Blocker technology to detect viral RNA and differentiate L and S-strain types.





