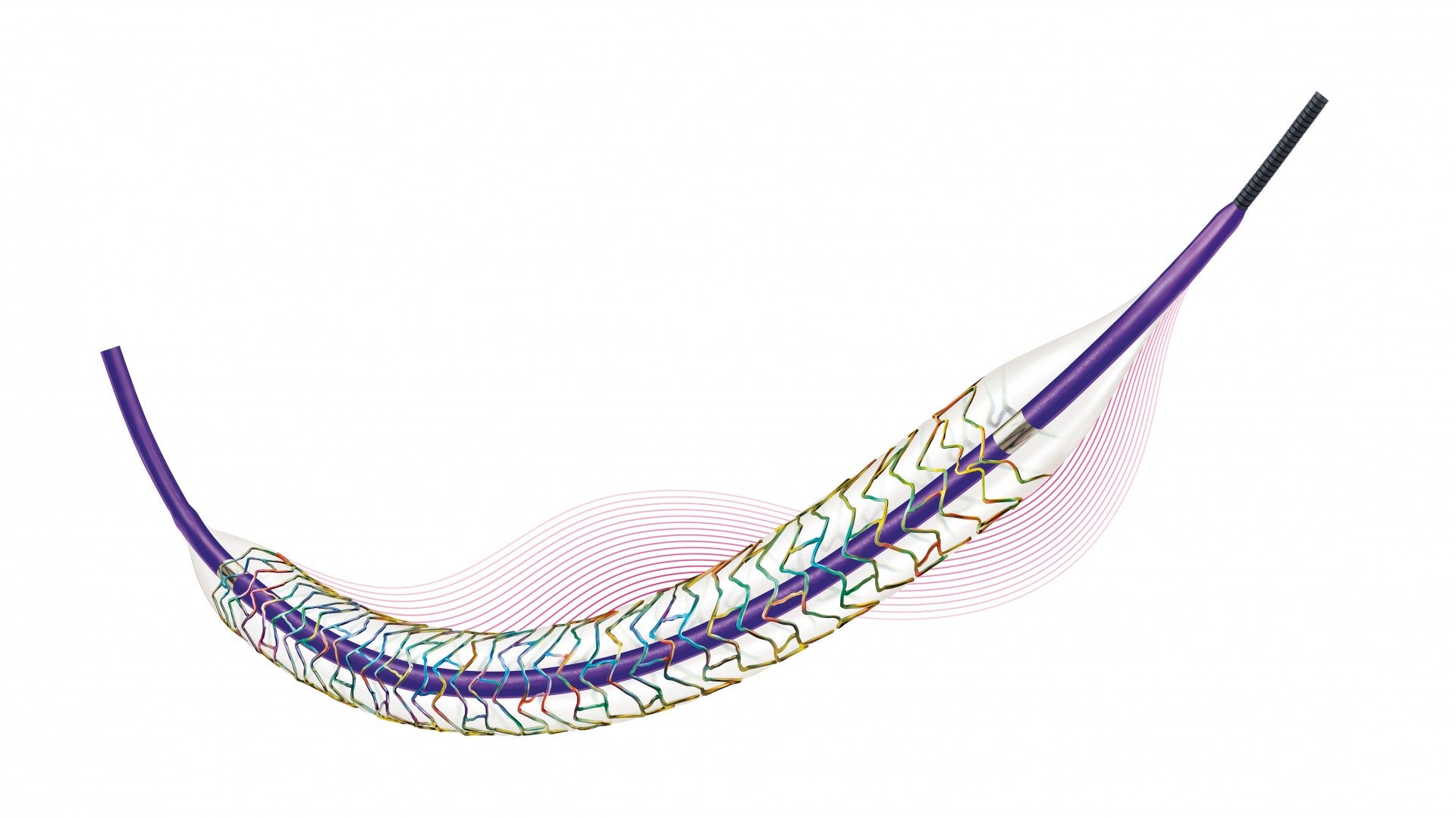
Biotronik has received approval from the US Food and Drug Administration (FDA) for its new Orsiro Mission bioabsorbable polymer drug-eluting stent system (BP-DES).
The company also announced the first implant and commercial launch of its new Orsiro Mission DES system in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The same proprietary stent design and bioabsorbable coating with controlled drug release present in the Orsiro stent are featured in the new system.
The ultrathin strut design of the new Orsiro DES is said to be the thinnest available in the US.
Offering enhanced deliverability, the system is claimed to be one of the most studied stents currently available throughout many randomised controlled trials (RCTs), registries and meta-analyses.
A re-engineered delivery system and new deep embedding process to improve deliverability, trackability and crossability are added to the new Orsiro Mission DES system.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBiotronik chief medical officer David Hayes said: “We will continue to bolster our interventional cardiology portfolio with innovations like the Orsiro Mission and state-of-the-art PK Papyrus covered stent system to support and improve patient care.
“This emphasises our commitment to excellence and reinforces our vision to ensure the highest quality.”
Data obtained from the BIOFLOW-V three-year study conducted on 1,334 patients demonstrated that the new Orsiro DES system offered a 40% lower target lesion failure rate compared to Abbott’s Xience DES.
It also showed a 52% lower ischemia driven target lesion revascularisation rate and 46% lower target vessel myocardial infarction rate.
In a separate development, Abbott received approval from the US FDA for its Amplatzer Talisman PFO Occlusion System for the treatment of people with a patent foramen ovale (PFO) who are at risk of recurrent ischemic stroke.
The new Talisman system builds on the minimally invasive Amplatzer PFO Occluder, which has been used to treat more than 180,000 patients across the world.
It offers an additional 30mm device size as well as the pre-attached delivery cable featured in all Talisman PFO occluders to reduce preparation time for doctors.
Furthermore, the FDA has cleared the Amplatzer Talisman Delivery Sheath, which is used to deliver the occluder during implantation.





