US-based orthobiologics company Bioventus has decided to proceed with a previously agreed option structure to advance with its plans to acquire CartiHeal, developer of the Agili-C implant.
As decided, Bioventus will make a $50m escrow payment to demonstrate its indication to acquire CartiHeal.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The decision comes after Bioventus reviewed a statistical analysis report of the pivotal clinical trial of the Agili-C implant, reimbursement coding analysis and market diligence related to commercialisation opportunities.
Notably, CartiHeal secured a $15m investment from Bioventus last year.
Both companies also agreed to an option structure under which Bioventus would acquire CartiHeal if the latter secured US Food and Drug Administration (FDA) approval for the Agili-C implant.
Bioventus can execute the potential transaction by exercising a call option provided under the option agreement. The agreement also provides CartiHeal with a put option, which can be exercised following premarket approval (PMA) of Agili-C implant by the FDA.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAfter such option rights are exercised, the completion of the transaction is subject to certain customary conditions.
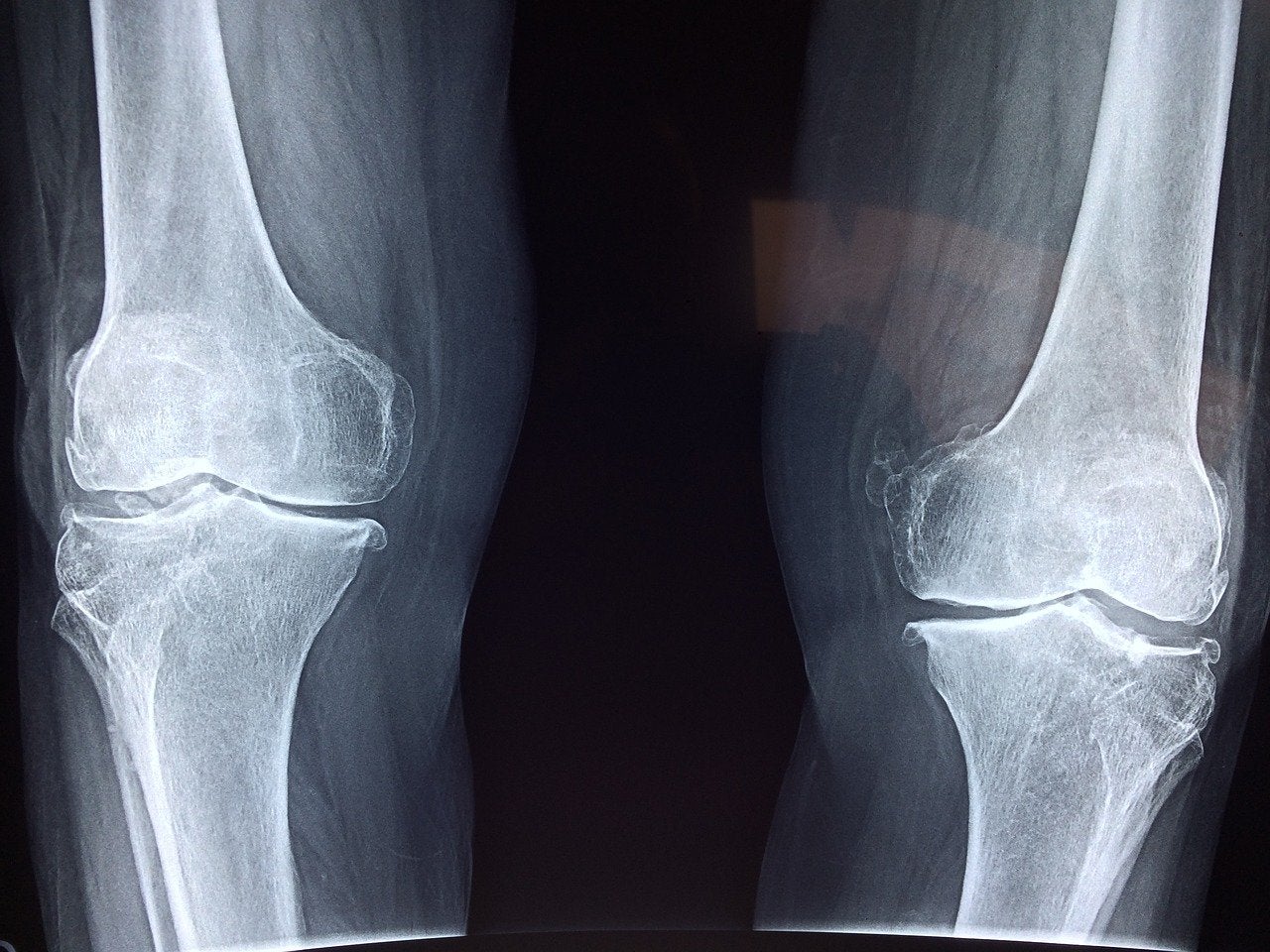
Israel-headquartered CartiHeal developed the Agili-C implant for treating cartilage and osteochondral defects in the knee joints of patients without severe osteoarthritis.
Bioventus senior vice-president and chief science officer Alessandra Pavesio said: “The robust data generated from the pivotal clinical trial, a randomised controlled trial with Agili-C, demonstrated superiority over the surgical standard of care, microfracture and debridement, in knee injury and osteoarthritis outcome score (KOOS) overall compared to baseline.
“We believe this product could be a strong alternative for the approximately 650,000 US patients annually receiving microfracture or debridement along with other cartilage treatment options.
“In combination with our hyaluronic acid (HA) products, Agili-C represents an exciting potential new offering for our portfolio designed to address the spectrum of osteoarthritis disease.”
The Agili-C implant received FDA Breakthrough Device Designation in 2020. CartiHeal now plans to submit the clinical module of their PMA later this year.





