Boston Scientific has entered into an agreement to purchase medical devices developer Claret Medical for an upfront cash payment of $220m.
The terms of the agreement additionally include up to $50m as a potential reimbursement-based milestone payment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
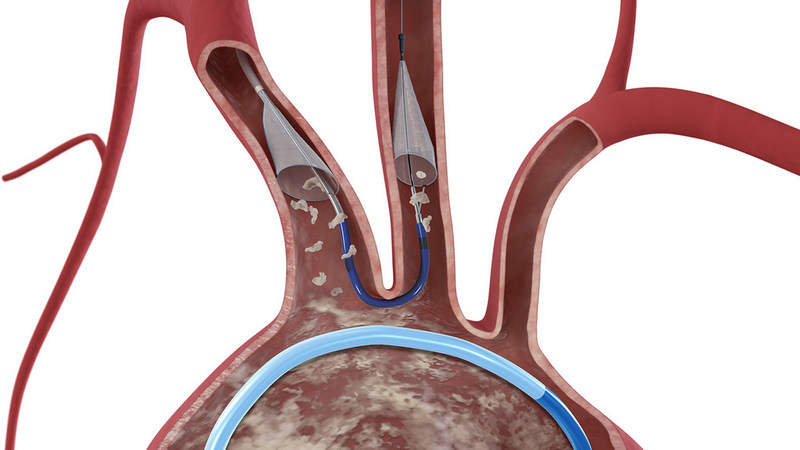
The Claret Medical portfolio includes the Sentinel Cerebral Embolic Protection System intended to address the risk of stroke in patients undergoing transcatheter aortic valve replacement (TAVR).
The device has been designed to protect the brain in some interventional procedures performed during TAVR.
It gained the European CE-Mark in 2014, followed by the US Food and Drug Administration (FDA) approval last year. The acquisition forms part of Boston Scientific’s strategy to expand its structural heart offerings.
Boston Scientific Interventional Cardiology president Kevin Ballinger said: “Through the development and commercialisation of the Sentinel System, Claret Medical has successfully introduced a new layer of safety and peace of mind for physicians and their patients undergoing TAVR procedures.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“This acquisition will expand our commercial portfolio to include an important adjunctive offering aimed at improving TAVR patient outcomes.”
The Sentinel System is expected to be potentially useful in other left heart and endovascular procedures, including mitral valve repair and replacement, and left atrial appendage closure.
TAVR is a minimally–invasive procedure during which embolic debris such as calcium or tissue can break loose and travel through the bloodstream to the brain, leading to potential neurological and neurocognitive damage.
In clinical studies, the Sentinel System demonstrated an ability to capture debris flowing towards the brain in 99% of TAVR cases, irrespective of the type of replacement valve used.
The FDA approval of the device was based on the SENTINEL trial data that showed a 63% decrease in stroke incidence within the initial 72 hours of the procedure.





