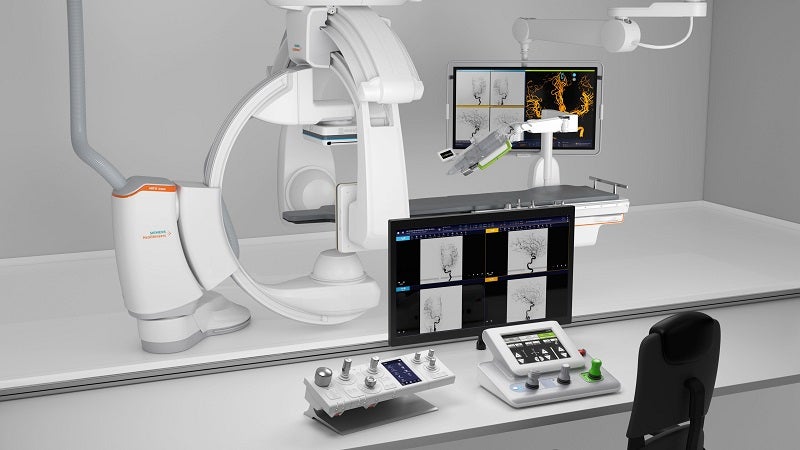
Siemens Healthineers company Corindus has reported positive data from the study of its CorPath GRX Neurovascular System used in robotic-assisted neurovascular aneurysm embolisation.
The multi-centre, single-arm, non-inferiority, prospective, global study is claimed to be the first trial in the world to be conducted on robotic-assisted neurovascular aneurysm embolisation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It included 117 participants from ten clinical locations in six different countries, along with a broad range of aneurysms from cases with different sizes, locations and morphology characteristics.
The findings from the study showed the effectiveness and safety of using the CorPath GRX Neurovascular System in robotic-assisted neurovascular aneurysm embolisation.
In the study, CorPath GRX showed a technical success of 94% and clinical success of 95.7%.
It also achieved its primary effectiveness goal, defined as successful robotic-assisted endovascular procedure completion.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAdditionally, CorPath GRX achieved the primary safety goal, which was defined as patients that were treated without intra- and periprocedural adverse events, such as vessel perforation or dissection, target aneurysmal rupture and thromboembolic event with neurological decline, within 24 hours of the procedure or discharge from the hospital.
In the study, 64.5% of the participants achieved Class I status.
This means they experienced complete obliteration of the aneurysm, on the Raymond-Roy Occlusion Classification (RROC).
Furthermore, 78.2% of the participants did not show any clinical symptoms after the procedure, while 21.8% of them had one or two Modified Rankin Scale (mRS).
Corindus Neuroendovascular chief medical officer Dr Raymond Turner said: “The work of our clinical partners on this study marks an early step toward truly transformative change in neurovascular intervention.
“By incorporating robotic platforms in this space, we are paving the way for remote interventional procedures in the future that will connect patients to specialised interventionalists for treatment, regardless of location.
“Validating clinical evidence, such as this study, will serve as the foundation for that transformation.”
The company claimed that the CorPath GRX System is the first FDA-approved and CE marked medical device designed for percutaneous coronary and vascular procedures.
It secured the CE mark for neurovascular procedures and is currently used for neurovascular interventions by healthcare facilities located outside the US.
At present, Corindus is seeking additional regulatory approvals for the CorPath GRX System for neurovascular indication.





