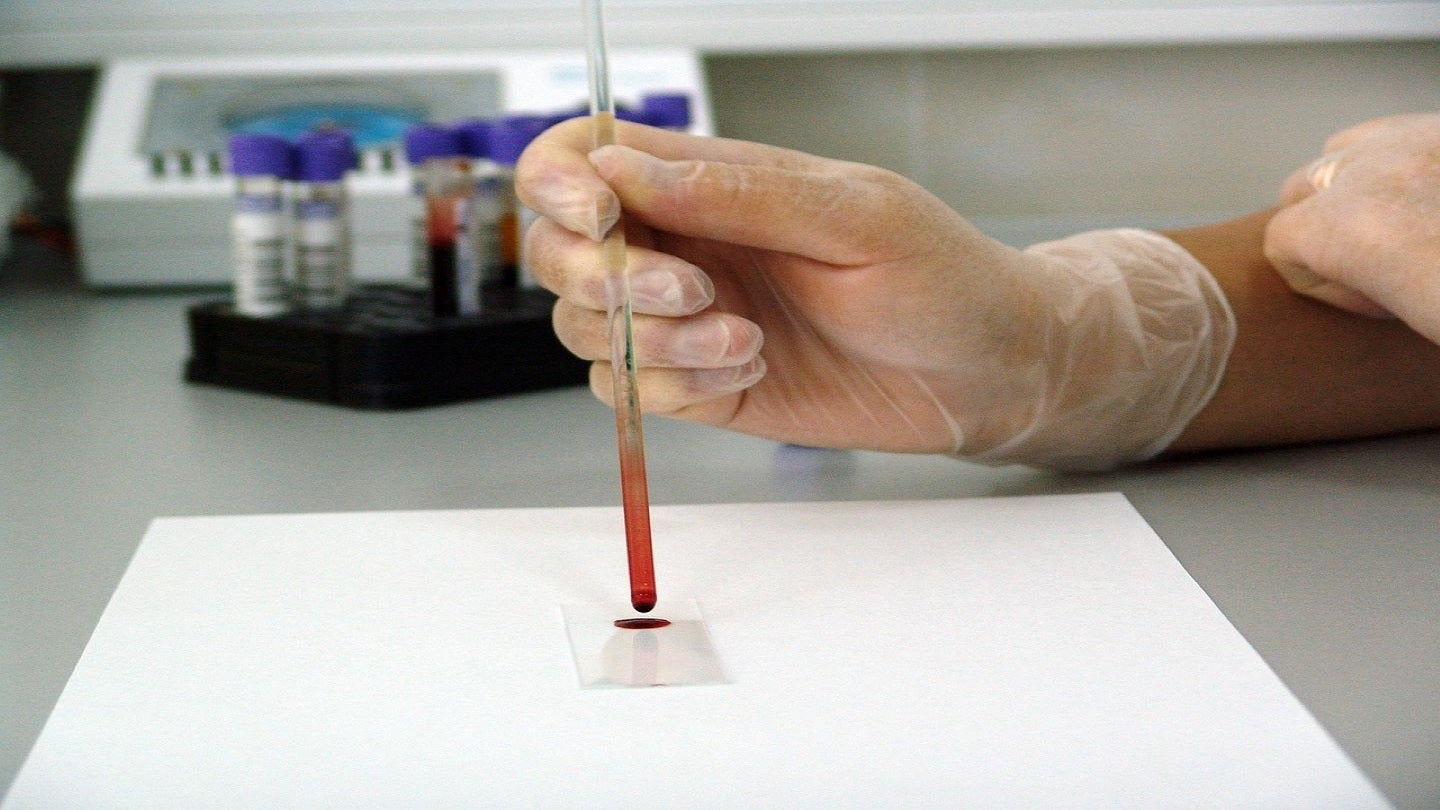
Medical diagnostic company Cytovale has commercially introduced its new diagnostic tool, the IntelliSep sepsis test, in the US.
The test was launched following 510(k) clearance from the US Food and Drug Administration in December last year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
With the capability to deliver results within ten minutes, the test enables quick diagnosis of sepsis for adults with signs and symptoms of infection.
It divides patients into three bands based on their sepsis probability using a standard blood sample.
The results could offer providers a tool for enhancing clinical outcomes and enable hospitals to better utilise resources, leading to operational improvements.
Cytovale co-founder and CEO Ajay Shah said: “The launch of the IntelliSep test is a huge moment in our fight against the devastating impact of sepsis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We know early detection and diagnosis is key and we’re proud to have a partner in Our Lady of the Lake and look forward to seeing IntelliSep’s impact at other Franciscan Missionaries of Our Lady Health System (FMOLHS) hospitals.”
Part of FMOLHS, Our Lady of the Lake Regional Medical Center in Baton Rouge, Louisiana, has become the first US medical centre to implement IntelliSep for its emergency department.
Cytovale aims to expand the reach of IntelliSep across the wider FMOLHS network and an extended clinical network through its collaboration with Louisiana State University.
In addition, the company is negotiating with other US hospital systems to expand the reach of the test.





