French medTech company eCential Robotics has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its spine surgery platform.
The company aims to get access to its 3D imaging, navigation, and surgical robotics guidance system in the North American market, following its recently established collaborations with implant companies in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
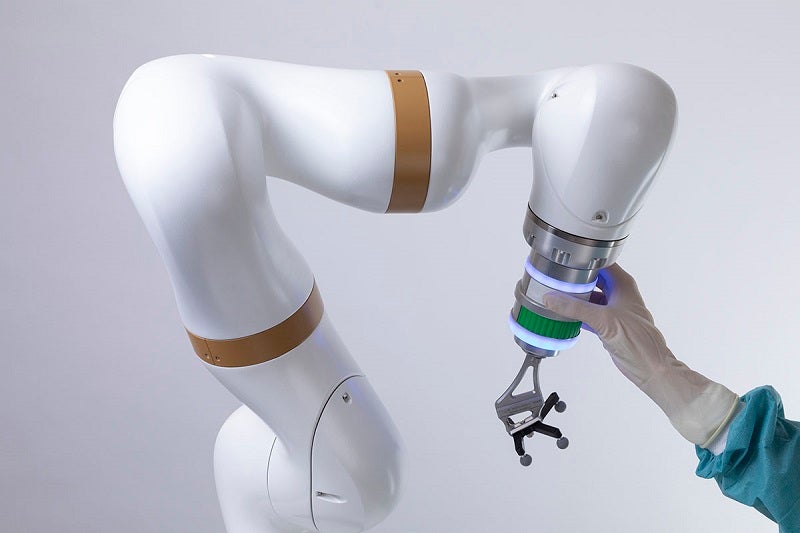
The surgical platform’s core design combines the functions of intraoperative 2D/3D imaging, navigation, and robotics.
According to the company, the unified open platform avoids the registration steps and unreliable calibration and simplifies the surgical workflow by automating several technical steps.
It has a single user interface for all the devices to control the imaging, navigation and robotic functions from one input and output graphical display screen.
The system comprises three mobile interconnected units that include a mobile viewing workstation, a mobile C-arm, and a mobile collaborative robot.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataeCential Robotics chief strategy officer and US CEO Laurence Chabanas said: “The FDA clearance of the eCential Robotics unified platform recognises reliability and robustness of our product, confirms the confidence in eCential Robotics’ unique concept of focusing surgical workflows on the essential via a unified, open, and multi-app system and also encourages our ambition to expand our footprint in the US.
“We are excited about these bold new and disruptive technologies and the role that eCential Robotics can play in reshaping bone surgical procedures and restoring patients’ quality of life.”
The company has obtained CE mark approval and commercialised its first platform in France, after several years of development of unique technology and workflow.
It has sold and installed ten systems in Europe, with more than 2,000 surgeries performed using them.
In December 2020, eCential Robotics created a subsidiary to support its efforts to enter the North American market.
In the US, the company conducted pre-clinical evaluation tests with neurosurgeons and orthopaedic surgeons in the first quarter of this year.





