Surgical guidance firm Endomag has announced that the US Food and Drug Administration (FDA) has granted premarket approval for the company’s breast cancer device Magtrace, the first non-radioactive dual-tracer for lymphatic mapping in breast cancer patients.
Magtrace, together with the Sentimag Magnetic Localization System, enables the use of magnetic detection during sentinel lymph node biopsy procedures to identify sentinel lymph nodes for surgical removal.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The breast cancer device has been designed to help US hospitals reduce their use of radioactive tracers, reducing the exposure of staff and patients to radioactivity and allowing surgeons to perform lesion localisation and sentinel node biopsy with a single device.
University of California, San Francisco (UCSF) professor of surgery Michael Alvarado is the principal investigator of the US Magtrace trial. He said: “We’ve been watching this technology become established in Europe over the past few years, and have been eagerly awaiting its availability in the US.
“After 18 months of using Endomag’s Sentimag platform with their Magseed marker for lesion localisation, we’re really excited to add the sentinel node biopsy capability with Magtrace. Being able to carry out both seed localisation and sentinel node biopsy in one box made this the only option for us. Magtrace and Magseed not only help to reduce the hospital staff and patients from exposure to radioactivity, they also offer us flexibility and so many more options when deciding on how to approach our breast cancer patients.”
The current favoured treatment for breast cancer patients is the surgical removal of the tumour and then a lymphatic mapping procedure to determine whether or not the cancer has spread to other parts of the body. This usually involves the use of radioactive drugs and blue dyes, which have a limited availability, can cause scheduling delays and are known to have very painful side effects. The Magtrace system is a non-radioactive alternative to current procedures and has been developed to improve this process for patients and surgeons.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
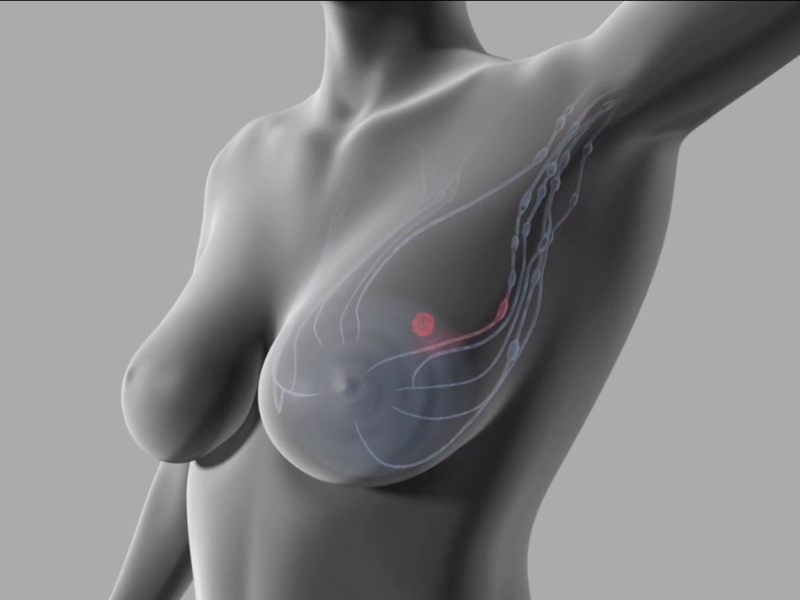
By GlobalDataThe collection of the Magtrace in these nodes allows surgeons to accurately target them for removal, without disrupting other nodes in the region. This is critical in determining the tumour stage and deciding on the best treatment pathway for the patient.
The breast cancer device is a dual-tracer consisting of magnetic fluid with iron oxide particles that are designed to follow the most likely route of cancer cells when they spread across a patient’s body. The liquid can be injected during an operation or up to a week before surgery as the trace is small enough to move rapidly through breast but big enough to be filtered by the first draining of entinel lymph nodes.
Endomag CEO Eric Mayes said: “This achievement demonstrates our team’s ability to innovate for the benefit of clinicians and their patients.
“The Sentimag platform is the first and only non-radioactive solution approved in the US that can perform lesion localisation and sentinel node biopsy. These two devices offer clinicians greater options and more flexibility when treating their patients and will help transform the way breast cancer is treated in the US.”
Magtrace will be exclusively distributed in North America by Leica Biosystems, the current market leader for sentinel lymph node biopsy.





