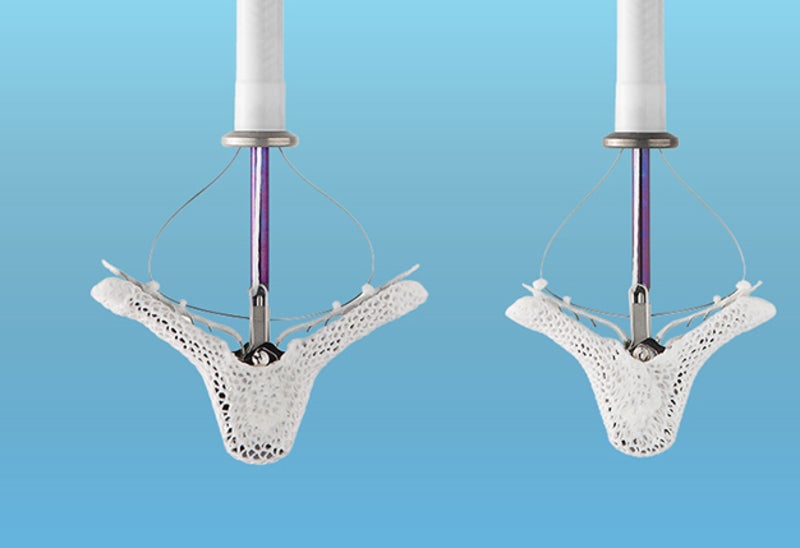
The US Food and Drug Administration (FDA) has approved Abbott’s MitraClip G4 heart valve repair device for the treatment of patients with mitral regurgitation.
MitraClip G4 can be delivered to the heart via an incision in the leg. The device is intended to repair leaky mitral valves without the need for open-heart surgery.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It clips together the leaflet portions of the mitral valve in order to minimise the backflow of blood and restore the heart’s ability to pump oxygenated blood.
The latest fourth-generation device is said to feature new clip sizes and an improved leaflet grasping capability. It comes with independently controlled grippers to facilitate grasping of one or both leaflets.
Moreover, MitraClip G4’s upgraded catheter enables continuous monitoring of left atrial pressure in real time during the implant procedure. This is intended to help doctors decide positioning of the device for improved patient outcomes.
Abbott structural heart business chief medical officer Neil Moat said: “We are continually innovating the MitraClip technology based on the experience of the physicians implanting the device so we can truly help them improve the lives of their patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“With the fourth generation of MitraClip, we set out to build a system that would help physicians individualise the therapy to each patient and deliver even more features that can treat both primary and secondary mitral regurgitation.”
Abbott initially launched the MitraClip system in Europe in 2008 and in the US in 2013. To date, the device has been used to treat primary and secondary mitral regurgitation in more than 80,000 patients globally.
The FDA approved MitraClip for primary mitral regurgitation in 2013. The device secured regulator approval for secondary mitral regurgitation in March.





