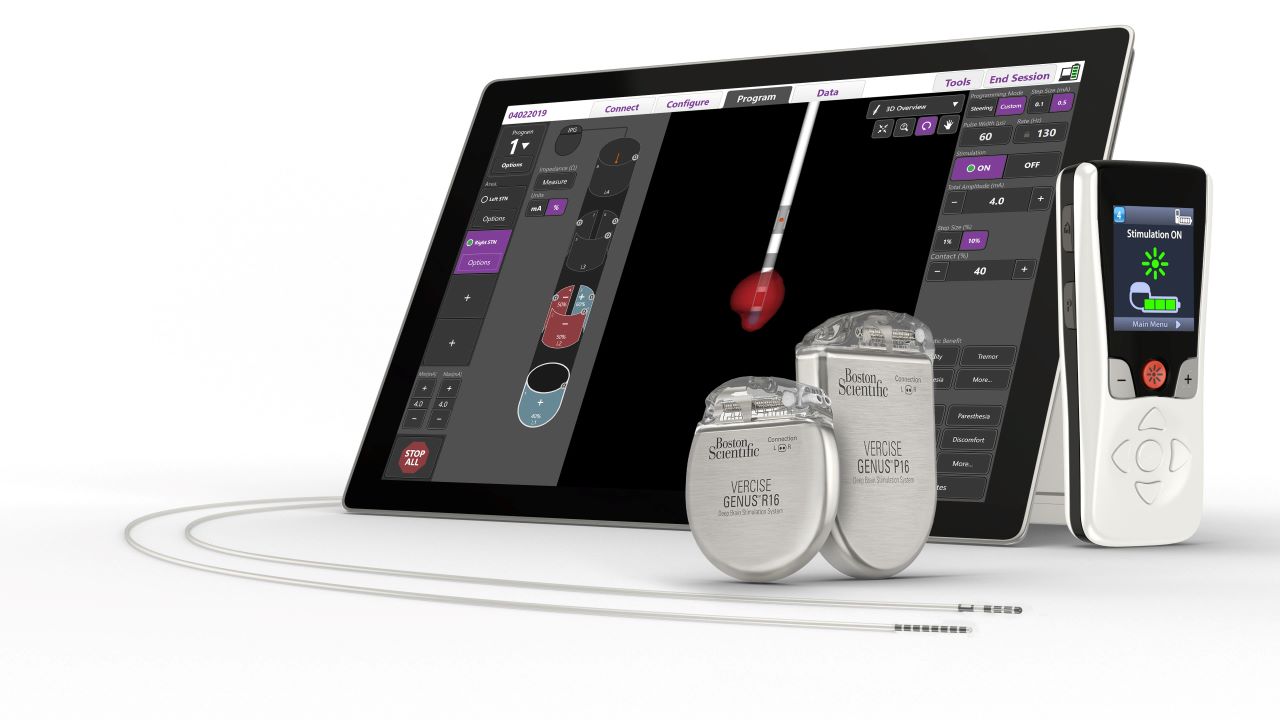
The US Food and Drug Administration (FDA) has approved Boston Scientific’s fourth-generation Vercise Genus Deep Brain Stimulation (DBS) System for conditional use in a magnetic resonance imaging (MRI) environment.
The portfolio has a family of Bluetooth-enabled, rechargeable and non-rechargeable, implantable pulse generators (IPGs) that power Cartesia Directional Leads designed for optimal symptom relief.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Boston Scientific noted that more than ten million people across the world are living with Parkinson’s disease (PD). A progressive, neurodegenerative disorder, it causes stiffness, slowness, and tremors because of reduced dopamine levels in the brain.
DBS devices such as Vercise Genus System can treat PD symptoms by providing targeted electrical stimulation though surgically implanted leads in the brain connected to an IPG.
Indicated for use in the bilateral stimulation of the subthalamic nucleus (STN), the system is used as an adjunctive therapy in reducing some symptoms of moderate to advanced levodopa-responsive PD not adequately controlled with medication.
Furthermore, it is also indicated for use in the bilateral stimulation of the internal globus pallidus (GP) as an adjunctive therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBoston Scientific Neuromodulation senior vice-president and president Maulik Nanavaty said: “We continue to prioritise therapy innovations that improve our patients’ quality of life with a wide range of personalised offerings.
“For people living with movement disorders, this means developing new technologies that are designed to refine motor control, reduce programming times, and expand MR compatibility to improve their treatment experience, and ultimately, their daily living.”
Last September, the company initiated the European launch of the Vercise Genus System and plans to start a controlled US launch soon.
This month, Boston Scientific announced a limited market release of its WaveWriter Alpha portfolio of Spinal Cord Stimulator (SCS) systems.





