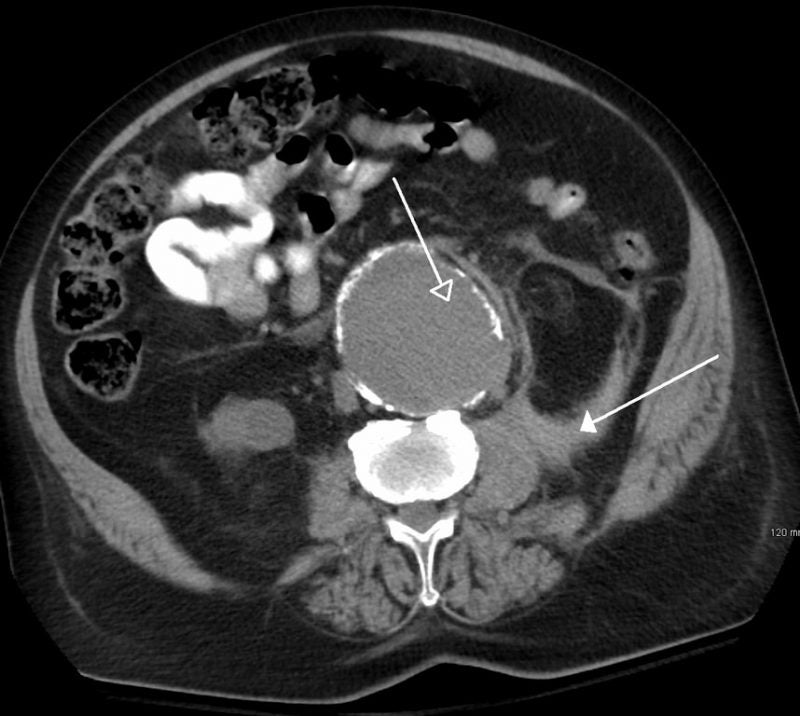
The US Food and Drug Administration (FDA) has granted breakthrough device designation to Endologix’s Chimney EndoVascular Aneurysm Sealing (ChEVAS) System for treating abdominal aortic aneurysm (AAA) patients.
An investigational endovascular AAA sealing therapy, the ChEVAS System integrates the Nellix 3.5 endograft with parallel visceral stents for the treatment of juxtarenal, pararenal and suprarenal AAA.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
At present, the system is being analysed in the ChEVAS ONE Investigational Device Exemption (IDE) clinical study.
This trial will enrol nearly 120 subjects at up to 50 clinical centres globally.
Endologix chief medical officer Matt Thompson said: “The ChEVAS System represents an important therapy that provides an ‘off-the shelf’ treatment to an underserved patient population who have complex abdominal aortic aneurysms.
“The ChEVAS System joins the PQ Bypass DETOUR System as the two FDA designated breakthrough devices in our clinical investigational programmes, which is reflective of our aspiration to provide innovative and disruptive technologies to address clinically relevant therapeutic gaps.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn April, Endologix acquired medical technology company PQ Bypass to bolster vascular reach to treat unmet medical needs of both AAA and peripheral arterial disease patients.
Separately, the US FDA has approved Nevro’s Senza System to treat chronic pain linked to painful diabetic neuropathy (PDN).
The latest approval is for the company’s 10kHz Spinal Cord Stimulation (SCS).
Nevro plans to commence commercial launch activities in the US under its recently unveiled HFX branding, HFX for PDN.
Nevro chairman, CEO and president D Keith Grossman said: “This FDA approval marks a capstone achievement that demonstrates the strength of our clinical data and provides a proven, new breakthrough SCS treatment option for PDN patients who are struggling with debilitating pain and who are unable to find relief with currently available pharmacologic options.”
In the randomised controlled SENZA-PDN trial, treatment with 10kHz SCS demonstrated enhanced and sustained outcomes in participants, including pain relief and improved health-related quality of life.





