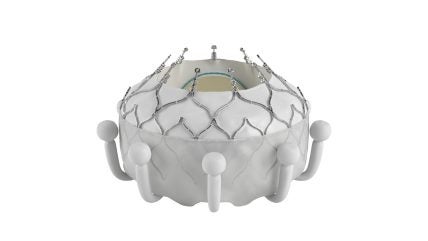
Edwards Lifesciences has received approval from the US Food and Drug Administration (FDA) for its EVOQUE tricuspid valve replacement system, a transcatheter therapy, for treating TR.
The system has been designed for patients with symptomatic severe TR who have not responded to optimal medical therapy and for whom valve replacement is considered appropriate by a heart team.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It features a nitinol self-expanding frame, an intra-annular sealing skirt, and tissue leaflets crafted from Edwards Lifesciences’s bovine pericardial tissue.
To be available in three sizes, the valve will be delivered through a low-profile transfemoral 28F system.
Edwards Lifesciences transcatheter mitral and tricuspid therapies corporate vice-president Daveen Chopra said: “Edwards has a long history of leading innovation and pioneering new therapies to address the unmet needs of patients with structural heart disease.
“We are grateful for the strong collaboration with clinicians all over the world who contributed to the EVOQUE system now being available through FDA’s Breakthrough Pathway to provide a treatment option to the many patients in the US suffering from tricuspid valve disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTRISCEND II trial, a six-month long study, showcased the system’s success, with results presented at Transcatheter Cardiovascular Therapeutics (TCT) 2023.
The trial demonstrated the EVOQUE system’s superiority over optimal medical therapy alone, achieving all primary endpoints.
Significant reductions or eliminations of TR and sustained quality of life improvements were noted, alongside a favourable risk-benefit profile.
Further data from the TRISCEND II trial, involving 318 out of 392 randomised patients who completed a one-year visit, indicated positive trends in the device group versus the control group.
The primary composite endpoints included all-cause mortality, heart failure hospitalisation, tricuspid intervention and improvements in the Kansas City Cardiomyopathy Questionnaire (KCCQ), New York Heart Association (NYHA), and six-minute walk distance (6MWD).
The EVOQUE system also received CE mark approval last year, claiming to be the first transcatheter valve replacement therapy globally approved for the treatment of TR.





