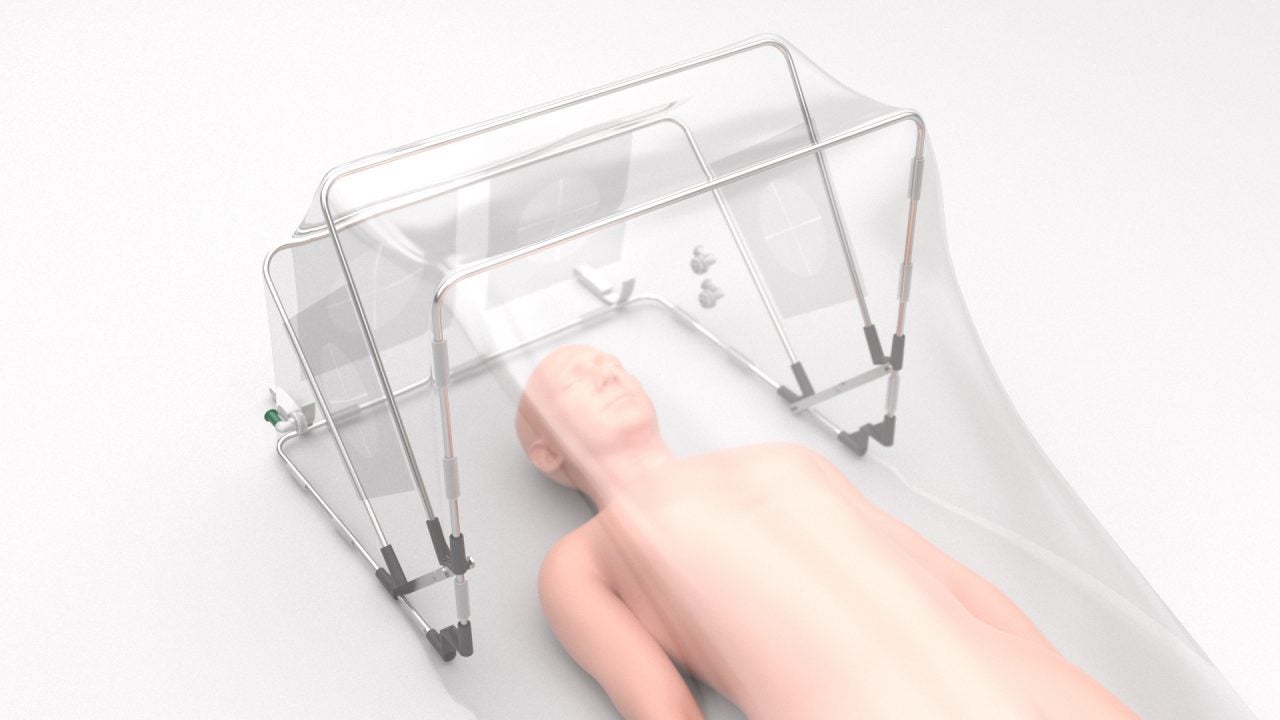
The US Food and Drug Administration (FDA) has granted Emergency Use Authorisation (EUA) to SCONE Medical Solutions’ Self-Contained Negative Pressure Environment (SCONE).
Using negative pressure technology, the SCONE device provides ‘active’ barrier protection and helps hospitals to lower the risk of infectious aerosol exposure to healthcare workers.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Apart from reducing the spread of transmissible diseases, the disposable, low cost and easy to use device can boost hospital throughput and enhance the quality of patient care.
SCONE Medical Solutions designed and developed the device in partnership with medical device developer Gilero and clinical support from Mayo Clinic.
SCONE Medical Solutions CEO Mike Adams said: “Our choice to partner with Gilero for the design and development of the SCONE device has given us a clear advantage in our speed to market.
“The SCONE device is a new kind of PPE that provides an additional layer of protection and opens up new possibilities for hospitals to triage and transport patients safely and efficiently through the ER and ICU.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn pre-clinical testing, the SCONE achieved a five-minute clearance rate under expected operating conditions.
Gilero Business Development director Clayton Roberts said: “In as little as four months, Gilero was able to refine SCONE’s original design concept and develop an innovative product that was functional, easy to use, and designed for manufacturability.
“Gilero is also manufacturing this product at our facility in Pittsboro, NC. The materials needed to manufacture this device are in high demand because of Covid-19, so we had to get creative and leverage our knowledge of the supply chain to source the raw materials needed.”
The disposable SCONE device is now available for sale to hospitals in the US.





