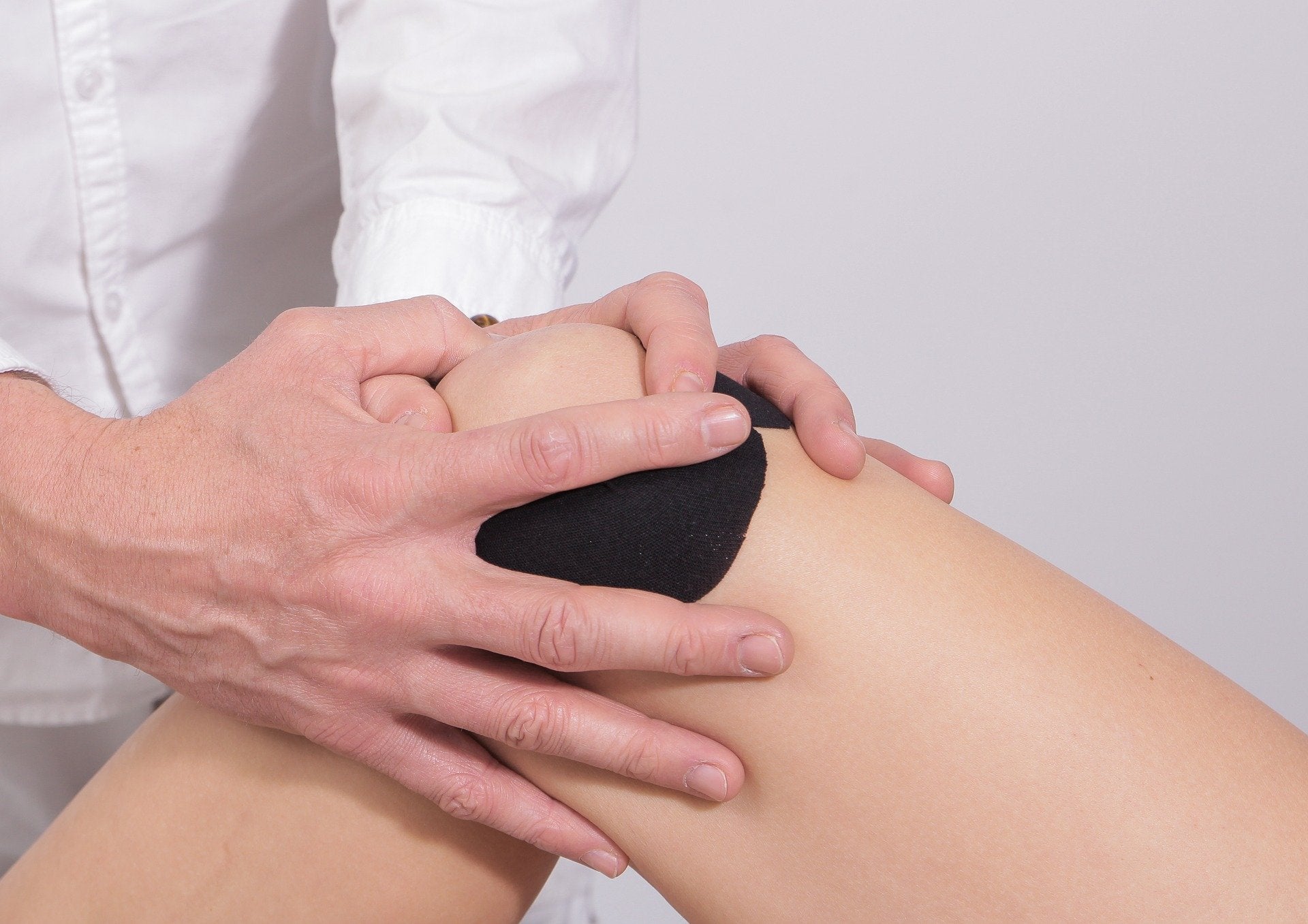
The US Food and Drug Administration (FDA) has granted breakthrough device designation to Siemens Healthineers company Varian’s Embozene microspheres for genicular artery embolisation (GAE) for symptomatic knee osteoarthritis.
The medical device obtained the status due to its potential to treat knee osteoarthritis patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Intended for lowering blood flow to the periarticular tissue of the joints, GAE prevents the inflammatory process.
Embozene is cleared by the FDA for the embolisation of arteriovenous deformities, uterine fibroids, hypervascular tumours and benign prostatic hyperplasia.
Varian Interventional Solutions president Frank Facchini said: “GAE holds great promise in providing clinicians with a new, non-invasive treatment option, which may not only ameliorate pain but reduce the economic burden of this common disease.
“Varian’s investigational programme for GAE exemplifies our commitment to investing in our core technologies to determine their potential to treat the world’s most debilitating diseases.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Breakthrough Device Program of the FDA offers patients prompt access to medical devices that can potentially deliver diagnosis or treatment for fatal or irreversibly debilitating diseases or ailments.
A common type of arthritis, osteoarthritis is a ‘wear and tear’ degenerative joint disease that causes an impact on the cartilage and adjacent tissues.
The knee is the joint that is most impacted with osteoarthritis, and knee osteoarthritis is a major cause of disability in adults in the US.
Estimates show that 14 million people in the US have symptomatic knee osteoarthritis, with 50% of them suffering from an advanced form of the disease.
In March, Varian Medical Systems made an investment in Bend It Technologies, an Israel-based company that is developing steerable microcatheters.
Bend It’s microcatheters are used for minimally invasive peripheral vascular procedures.





