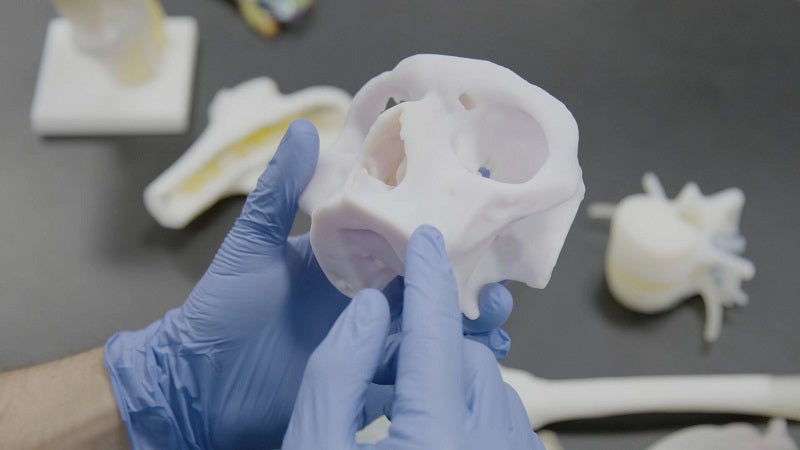
The US Food and Drug Administration (FDA) has granted 510(k) clearance for the Ricoh 3D for Healthcare craniomaxillofacial (CMF) and orthopaedic anatomic modelling.
An integrated, end-to-end workflow solution, Ricoh 3D for Healthcare is intended to develop, design and produce anatomic models using 3D printing technology from Stratasys.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Integrated with IBM iConnect Access, an enterprise imaging solution used in many US hospitals, the solution allows healthcare providers to create patient-specific representations of tissue and bone.
These models help clinicians view the inside anatomy of patients and provide precision medicine.
They can also be used for patient education, pre-surgical planning and anatomic simulation to practice new/rare approaches.
The company stated that healthcare providers can choose a workstyle option to suit their needs. With the point-of-care option, a services team managed by Ricoh will work onsite to oversee the complete process using Stratasys 3D printers.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe on-demand option allows providers to order models to be printed at a Ricoh facility and shipped directly to them.
Ricoh USA North America additive manufacturing managing director Gary Turner said: “Ricoh is committed to healthcare innovation that will turn the tide on patient engagement and precision medicine. Ricoh 3D for Healthcare does just that – offering a model matched to the unique anatomy of each individual patient.
“One of our goals is to address the needs of doctors and patients in specific areas with particularly high demand for 3D-printed anatomic models, the 510(k) clearance for CMF and orthopaedic models moves us further toward reaching this goal.”
The company plans to request 510(k) clearance for additional treatment regions in the future.





