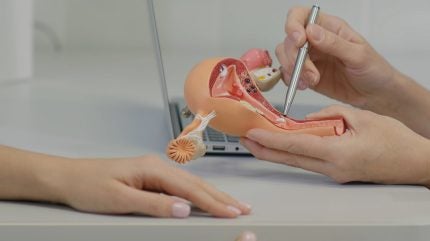
Biomedical company Femasys has secured CE mark certification and Health Canada approval for FemVue MINI, a compact solution for assessing fallopian tubes.
Designed for in-office use, the FemVue MINI device offers the same efficacy as the original FemVue but in a more environmentally friendly package.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The innovation aligns with Femasys’ commitment to sustainable healthcare solutions, as the device’s smaller size reduces its environmental footprint.
Femasys’ initiative to incorporate eco-conscious practices extends across its product lines.
The FemVue MINI is part of this broader effort to integrate impactful environmental programmes into the company’s offerings.
Femasys founder, president, and CEO Kathy Lee-Sepsick said: “Our mission at Femasys has always been to develop cutting-edge technology that are safe, affordable, and accessible to address critical needs in women’s health.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“With the FemVue MINI, we are advancing an equally effective solution in a smaller footprint that is aligned with our initiative to be environmentally conscious demonstrating our commitment to sustainability.”
FemVue is an FDA-cleared product that creates natural contrast for real-time ultrasound evaluation of fallopian tubes in a gynaecologist’s office.
It is a safer and less expensive alternative to traditional radiology exams.
FemVue is also approved for use in Canada and Europe.
In June 2024, Femasys obtained the EU Medical Device Regulation (MDR) certificate and CE Mark certification for four women’s health products.
The products include FemaSeed, FemVue, FemCerv and FemCath and are developed for women’s reproductive health.
The development paves the way for the company to commence commercialisation efforts for the products within the European market.





