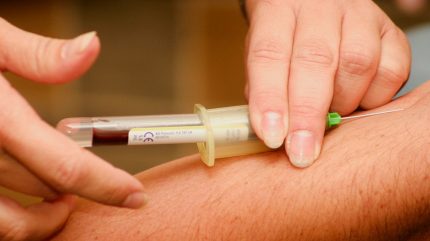
GRAIL has completed the planned enrolment of more than 35,000 subjects for the PATHFINDER 2 study, which is evaluating the Galleri multi-cancer early detection (MCED) test.
The study targets individuals aged 50 years and above in the US, who are eligible for guideline-recommended cancer screening.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The PATHFINDER 2 study, which began enrolling participants in December 2021, operates under an investigational device exemption approved by the Food and Drug Administration.
Its primary goals are to assess the safety and effectiveness of the MCED test and to evaluate its performance through various metrics, including positive and negative predictive values, sensitivity, specificity, and cancer signal origin prediction accuracy.
The secondary objectives of the study involve the analysis of guideline-recommended cancer screening procedures post-MCED test use and participant-reported outcomes.
These include measuring participants’ anxiety levels and satisfaction with the test.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe study is being carried out in collaboration with healthcare institutions such as Cleveland Clinic and Duke University Health System.
Additionally, GRAIL has concluded the third and final round of trial visits for the NHS-Galleri registrational trial, which saw the enrolment of more than 140,000 participants aged between 50 and 77.
Initiated in 2021, this trial is a prospective, randomised, controlled clinical utility trial aimed at assessing the Galleri test in reducing the incidence of late-stage cancers through early detection.
GRAIL CEO Bob Ragusa said: “Both studies were designed to enrol a diverse participant population, representative of socioeconomic, ethnicity, gender and age differences, and we are proud of the diversity of the study populations.
“We look forward to seeing results from the first 25,000 individuals enrolled in the PATHFINDER 2 study in the second half of 2025 and final results from the NHS-Galleri trial in 2026.”
The NHS-Galleri trial achieved full enrolment in just over ten months. It requires participants to undergo blood draws over two years, with half receiving the Galleri test and the other half having their samples stored for analysis in the future.
Collaborators on this trial include Queen Mary University of London, and King’s College London Cancer Prevention Trials Unit, in partnership with NHS England.





