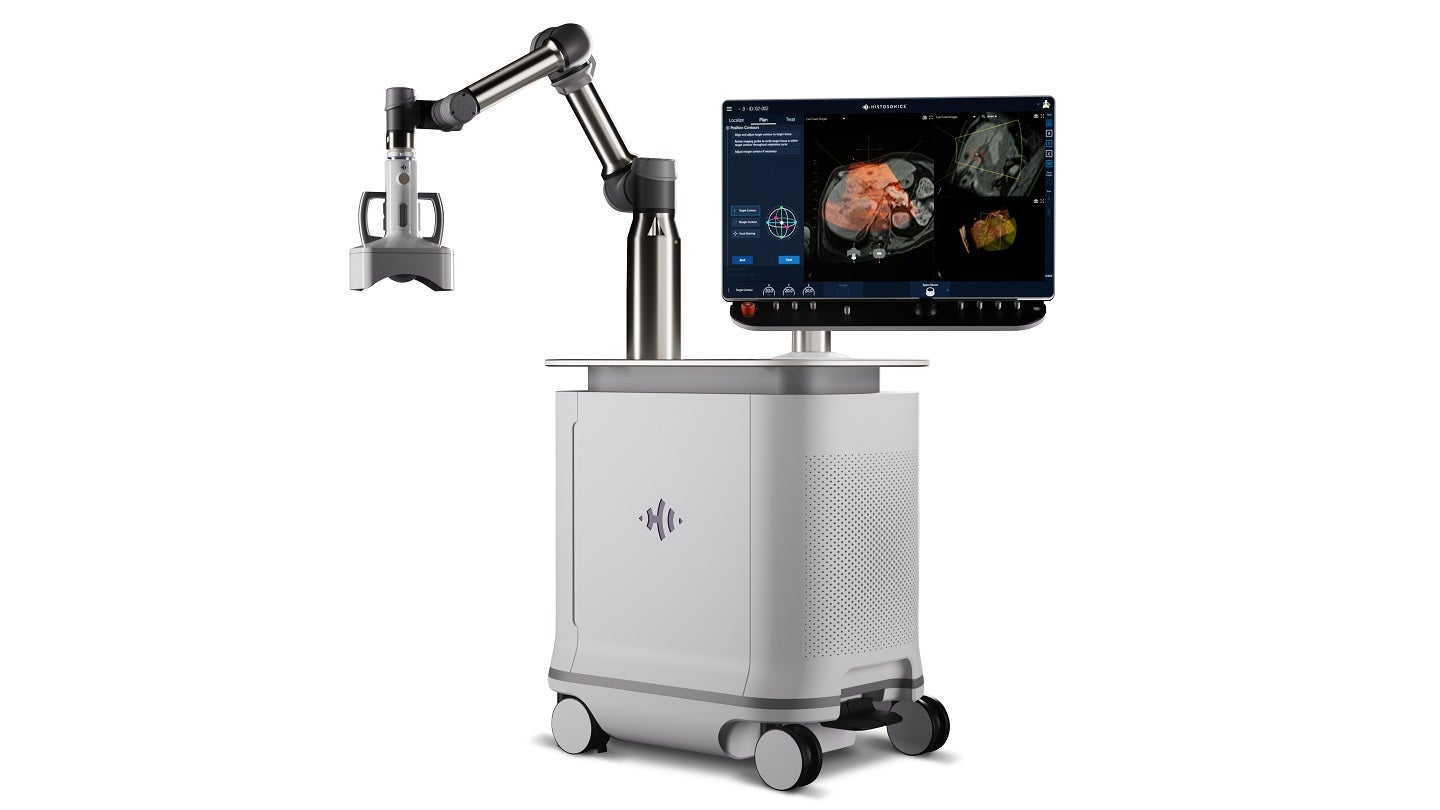
HistoSonics has received marketing authorisation from the US Food and Drug Administration (FDA) for its Edison histotripsy platform.
Approved through the De Novo Classification Request process, Edison is claimed to be the first and only histotripsy platform available in the country.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Edison system is intended for the non-invasive destruction of liver tumours, including unresectable liver tumours, utilising a non-thermal, mechanical process of focused ultrasound.
Leveraging technology and advanced imaging, it delivers personalised, non-invasive histotripsy treatments with control and precision.
The FDA authorisation was partially based on data from the #HOPE4LIVER Trials conducted across 13 trial sites in Europe and the US.
Data gathered from both European/UK and US studies were utilised to evaluate the clinical efficacy and safety of histotripsy in eliminating targeted primary and secondary liver tumours.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHistotripsy attained primary safety as well as efficacy endpoints in the pooled data, involving the assessment of safety in 44 subjects and the evaluation of efficacy in treating 44 tumours.
HistoSonics president and CEO Mike Blue said: “We have been thoughtfully adding professionals with deep domain experience in operations, market development and education and are prepared to begin scheduling physician training immediately.
“This is a fantastic day for patients who will benefit from the novel advantages of histotripsy, and I commend the FDA for working so expeditiously with us throughout the review process.”





