Imagen Technologies has received 510(k) clearance for Aorta-CAD, a computer-assisted detection (CADe) device, from the US Food and Drug Administration (FDA).
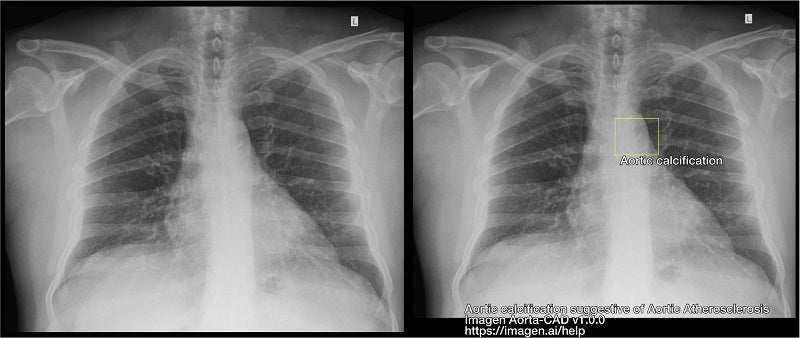
A cloud-based, software-only medical device, Aorta-CAD has been designed to help physicians detect chest X-ray findings that may indicate aortic atherosclerosis and aortic ectasia.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It leverages deep learning to spot and highlight findings that are related to aortic atherosclerosis and aortic ectasia, helping physicians, including radiologists and primary care physicians (PCPs), to read chest X-rays.
The company stated that the regulatory approval marks its fourth FDA clearance and will improve patient outcomes by making frontline imaging diagnostics suitable for PCP offices.
Aorta-CAD is intended to help identify previously undiagnosed ailments through the findings on chest X-ray images.
It creates annotations to draw attention to the findings through an overlay, which can be toggled off when not being used by the doctor.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe device can integrate into current chest X-ray workflows without requiring new picture archiving and communication system (PACS) technology or costly re-training.
It is said to be part of the company’s Diagnostics as a Service (DaaS) platform.
Imagen Technologies CEO Alex Dresner said: “Imagen is on a mission to help primary care physicians deliver faster, better diagnoses and care plans.
“By expanding our diagnostics as a service platform with Aorta-CAD, we expect to help PCPs significantly improve the quality of care and clinical outcomes for their patients with undiagnosed aortic atherosclerosis and aortic ectasia.”
The company expects that the addition of the Aorta-CAD device to its DaaS platform may lead to earlier and better detection and treatment for chronic diseases.
In its clinical trial, the device showed a 62% relative reduction in misses for aortic calcification suggestive of aortic atherosclerosis.
Aorta-CAD also showed a 35% relative reduction in misses for dilated aorta, which points to aortic ectasia, and 96% of physicians demonstrated improvement with its assistance.





