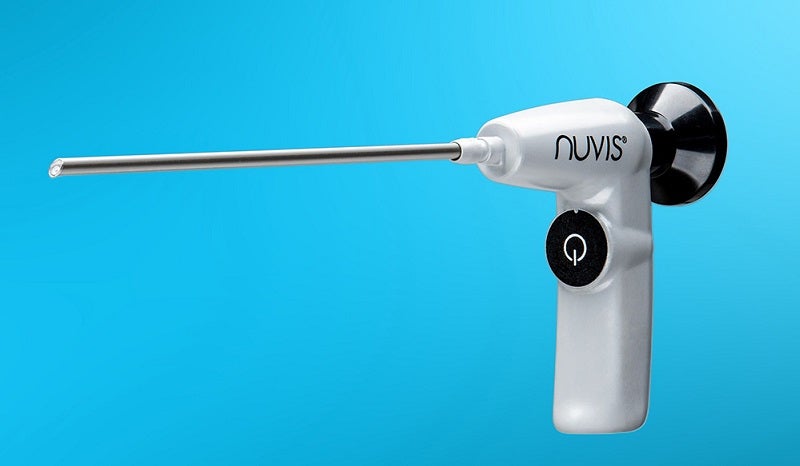
US-based medical device firm Integrated Endoscopy has launched its second-generation NUVIS single-use arthroscope worldwide.
The battery-operated, cordless endoscope has been designed for use in arthroscopic surgical processes to address issues facing conventional reusable arthroscopes.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It includes a patent-pending simplified optical train design that reduces the optical elements by more than 60% and improves image quality to 4K.
The Gen II NUVIS arthroscope features a built-in, battery-powered LED that eliminates the need for a light box or light cord.
Compatible with most installed video towers, it will also support a 4K camera system without the need for additional equipment.
Integrated Endoscopy said that the Gen II NUVIS arthroscope is less expensive to manufacture and amenable to high-volume automation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company is also introducing several versions of the NUVIS to make the technology compatible with most cannulas available on the market.
Integrated Endoscopy CEO Brad Sharp said: “The release of the Gen II NUVIS is a huge milestone for the company in terms of image quality and compatibility.
“Our nextgen arthroscope also provides a substantial decrease in manufacturing costs while simultaneously increasing ease of manufacturability.
“The demand we have seen for NUVIS far exceeds our current manufacturing capacity, so the efficiencies the Gen II NUVIS affords will greatly aid in shortening production time and costs.
“With a global market of over 1.5 million units per month, increasing our manufacturing capacity will be a major focus for the company moving forward.”
Integrated Endoscopy said the Gen II NUVIS is also CE-marked and has obtained 501(k) clearance from the US Food and Drug Administration (FDA).
The device is currently being registered in many other markets worldwide.
Integrated Endoscopy intends to expand its global distribution network as its manufacturing capacity increases.
Based in Irvine, California, the company has 19 issued patents in its intellectual property portfolio.





