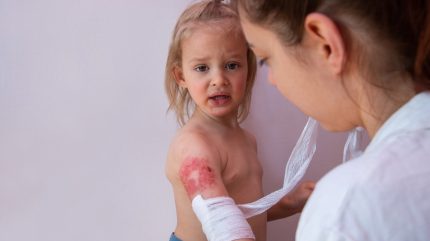
MediWound and Vericel Corporation’s thermal burn treatment, NexoBrid (anacaulase-bcdb) has been approved by the US Food and Drug Administration (FDA) for eschar removal in paediatric patients with deep partial-thickness and/or full-thickness thermal burns.
The current approval builds on MediWound’s previous approval for the same indication in the adult population in January. It generated $19m in sales last year, as per MediWound’s financials. GlobalData expects NexoBrid sales to maintain the upward trajectory and generate over $98m in sales by 2030.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
NexoBrid is a gel consisting of a sterile mixture of proteolytic enzymes, which selectively eliminate burn eschar within four hours without causing harm to surrounding viable tissue. It was developed by MediWound, with Vericel acquiring commercialisation rights to NexoBrid in North America in 2019.
The FDA approval for NexoBrid was based on the positive Phase III Children Innovative Debridement Study (CIDS) (NCT02278718). The trial enrolled 145 children under 17 years of age and compared NexoBrid with the current standard of care for burns. The gel removed unhealthy tissue in the burn area, allowing surgeons to properly gauge thickness and evaluate if surgical intervention is needed.
Another proteolytic enzyme gel in MediWound’s pipeline is EscharEx. The company recently published positive data from a Phase II ChronicEx trial (NCT03588130) showing its efficacy in treating venous leg ulcers. MediWound plans to start a Phase III trial evaluating EscharEx as a treatment for venous leg ulcers in the second half of this year. The company also plans to investigate EscharEx in another chronic wound condition, namely diabetic foot ulcers in the second half of 2025.
The wound care management market is expected to total $38.8bn by 2030, with a CAGR of 3.4%. Multiple companies have made advances in managing burns. Spectral AI is developing a diagnostics platform DeepView that can help predict wound healing outcomes and support clinical decision-making.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataKerecis has developed fish skin-derived tissue-transplant products for treating burns. Its Omega3 GraftGuide product is approved by the FDA for the management of chronic wounds, including diabetic, vascular, and other hard-to-heal wounds.





